
Our laboratory aims at elucidating basic processes taking place during embryonic development of the nervous and musculo-skeletal systems. We focus on four main subjects: first, the development of the peripheral nervous system (PNS) from the neural crest. Second, the cellular and molecular basis underlying the transition from the neural crest state (PNS) to the definitive roof plate of the CNS. Third, morphogens and mechanisms involved in muscle and vertebral development. Fourth, the molecular signals that mediate interactions between mesoderm and nervous system, found to be of significance for patterning and differentiation.Our lab has a rich academic record in avian embryology and molecular techniques that include in ovo microsurgery and grafting, targeted gene missexpression in embryos, in vitro explants and culture systems, imaging and RNAseq techniques.
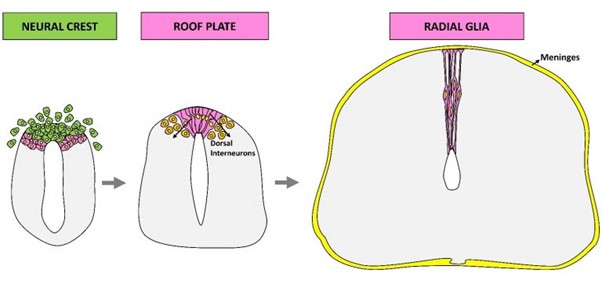
The development of the dorsal spinal cord is a highly dynamic process, starting with the neural tube harboring neural crest (NC) progenitors that generate the peripheral nervous system. This domain is then replaced by the roof plate (RP), a key patterning center for dorsal interneurons. Through dorsal collapse, the RP transitions into dorsal midline radial glial cells (dmRG). We are currently investigating the molecular mechanisms of these transitions.
In addition, we pursue experiments to understand the patterning of skeletal muscles and vertebrae, and the roles of morphogens such as Sonic hedgehog, etc in coordinating skeletal with neural development.
Our work focuses on avian embryos (chick and quail) and includes a variety of in vivo techniques, from tissue grafting to gene misexpression, in vitro explants, molecular profiling and imaging. We actively collaborate with researchers in the mammalian field to complement our knowledge using genetic models.
Research on the development of the dorsal neural tube is particularly challenging. In this highly dynamic domain, a temporal transition occurs between early neural crest progenitors that undergo an epithelial-to-mesenchymal transition and exit the neural primordium, and the subsequent roof plate, a resident epithelial group of cells that constitutes the dorsal midline of the central nervous system. Among other functions, the roof plate behaves as an organizing center for the generation of dorsal interneurons. Despite extensive knowledge of the formation, emigration and migration of neural crest progenitors, little is known about the mechanisms leading to the end of neural crest production and the transition into a roof plate stage.

image from: Rekler, D. and Kalcheim, C. (2021). From Neural Crest to definitive Roof Plate: the dynamic behavior of the dorsal neural tube. Int. J. Mol. Sci.
Through dorsal collapse, the RP transitions into dorsal midline radial glial cells (dmRG), which later give rise to the dorsal ependyma – a stem cell niche in the central nervous system. This project aims to investigate the spatio-temporal molecular and morphological changes driving this transition. We utilize selected genes from our recent single-cell RNA-seq dataset and time-dependent misexpression experiments.
The sonic hedgehog (Shh) protein plays fundamental roles in the development of the neural tube (NT) and somites. Following neurulation, Shh secreted by the notochord (No) induces distinct ventral cell identities in the overlying NT by a mechanism that depends on relative concentrations and duration of exposure. Moreover, the activity of Shh continues beyond this stage to regulate cell proliferation, survival and differentiation. No-derived Shh is also involved in mesoderm patterning.
In our lab we investigate the mechanisms of action of Shh in neural and mesodermal development.
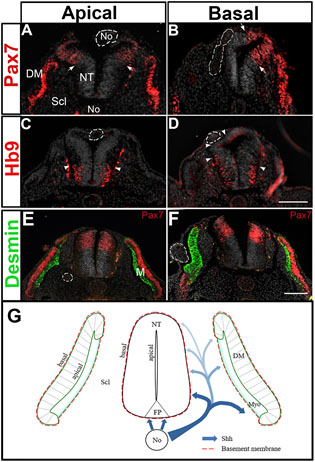
image from: Kahane, N. and Kalcheim, C (2020). Neural tube development depends on notochord-derived Sonic hedgehog released into the sclerotome. Development.
Our lab has been generously supported over the years by the Israel Science Foundation, DFG, IMRF, March of Dimes, ICRF and Familial Dysautonomia Foundation.
Rekler, D., Ofek, S., Kagan, S., Friedlander, G., & Kalcheim, C. (2024). Retinoic acid, an essential component of the roof plate organizer, promotes the spatio-temporal segregation of dorsal neural fates. Development, dev-202973.
Retinoic acid, produced by the dorsal neural tube in the developing embryo, plays a crucial role in ending the production of neural crest cells and initiating the formation of the roof plate. In this study, we investigate the separation of central and peripheral nervous system lineages, which is essential for proper tissue development and function. By locally inhibiting retinoic acid in quail embryos and using single-cell transcriptomics, we unraveled a comprehensive list of differentially expressed genes relevant to these processes.
We found that in the absence of retinoic acid, progenitor cells failed to properly segregate, simultaneously expressing markers for neural crest cells, roof plate cells, and dorsal interneurons (dI1). Further analysis revealed that Notch signaling, which acts downstream of retinoic acid, is critical for maintaining the boundary between the roof plate and dI1 interneurons.
Within the peripheral branch, where absence of retinoic acid resulted in neural crest production and emigration extending into the roof plate stage, sensory progenitors failed to separate from melanocytes leading to formation of a common glia-melanocyte cell with aberrant migratory patterns. Together, the implementation of scRNA sequencing facilitated the discovery and characterization of a molecular mechanism responsible for the segregation of dorsal neural fates during development.
Kahane, N., Dahan-Barda, Y., & Kalcheim, C. (2024). A Spatio-Temporal-Dependent Requirement of Sonic Hedgehog in the Early Development of Sclerotome-Derived Vertebrae and Ribs. International Journal of Molecular Sciences, 25(11), 5602.
Rekler, D. and Kalcheim, C. (2022). Completion of neural crest cell production and emigration is regulated by retinoic-acid-dependent inhibition of BMP signaling. eLife.
In this paper we reveal a role for neural tube-derived Retinoic Acid in ending the neural crest production. Retinoic acid acts through BMP signaling to downregulate neural crest-specific genes and to stop cell emigration from the neural tube. While it is also important for aspects of the development of definitive Roof Plate, not all Roof Plate traits depend on retinoic acid, suggesting that the end of neural crest production and the formation of the Roof Plate are in part independently regulated.
Ofek, S, Wiszniak, S, Kagan, S, Tondl, M, Schwarz, Q, and Kalcheim, C. (2021). Notch signaling is a critical initiator of roof plate formation as revealed by the use of RNA profiling of the dorsal neural tube. BMC Biol.
In this article we used RNA-seq to reveal differences in gene expression profile between Neural Crest and definitive Roof Plate cells. Based on these findings we proceed to show the importance of Notch signaling in the formation of the definitive Roof Plate of the spinal cord and highlight its role in the segregation between Roof Plate and dorsal interneurons.
Kahane, N. and Kalcheim, C (2020). Neural tube development depends on notochord-derived Sonic hedgehog released into the sclerotome. Development.
Recommended by Faculty Opinions (F1000).
In this article we reveal a role of the sclerotome as a site of Shh gradient, controlling development of mesodermal and neural progenitors.

Research associate
Early development of somites and muscles, and recently also ribs and vertebrae

Ph.D student
I am interested in the transition between the Neural Crest and the definitive Roof Plate in the developing nervous system.

MD-Ph.D student
The involvement of Delta-Notch signaling in roof plate development and in roof plate-interneurons border formation.

undergraduate student
Examining the effects of Shh deprivation in avian embryos using electroporation of mutated genes.
1973-1977: B.Sc. degree, Faculty of Life Sciences,Hebrew University of Jerusalem. Subjects: Physiology.
1977-1979: M.Sc. degree in Neuroendocrinology under the supervision of Prof. Y. Koch, Dept. of Hormone Research, The Weizmann Institute of Science, Rehovot.
1980-1984: Ph.D. degree under the supervision of Prof. Z. Vogel, Dept. of Neurobiology, The Weizmann Institute of Science, Rehovot.
Sept. 1984- Post-doctoral training in the laboratory of Prof. N. Le Douarin, Institut
Dec. 1986: d’Embryologie du C.N.R.S. et College de France, Nogent Sur Marne, France.
Jan. 1987- 1991 Lecturer in the Department of Anatomy and Embryology, Hebrew University- Hadassah Medical School. Jerusalem.
1992-1996 Senior Lecturer-
1996-2000 Associate Professor.
2001 – Present Professor. Department of Medical Neurobiology, IMRIC and ELSC-
2010 – Present Member of the ELSC, the Edmond and Lili Safra Center for Neurosciences of the Hebrew University.