
Associate Professor
With life expectancy increasing, neurodegenerative diseases are taking an ever-increasing toll on society. Several of these diseases are characterized by a selective degeneration of particular classes of neurons. Our laboratory focuses on understanding how the physiology of vulnerable neurons contributes to their degeneration as well as studying adaptive physiological responses to degeneration within neural networks. Our research focuses on Parkinson’s disease and Huntington’s disease. We study it using genetic and other models of the disorders. The lab has a special interest in studying the pathophysiology underlying the non-motor symptoms of prodromal PD, such as gastrointestinal dysmotility, anxiety & depression, chronic pain. We also are interested, more generally, in the cellular physiology of neurons and circuits in the Basal Ganglia. Our work combines neurophysiological experiments, advanced in vivo and in vitro imaging techniques and mathematical modeling.
With life expectancy increasing, neurodegenerative diseases are taking an ever-increasing toll on society. Several of these diseases are characterized by a selective degeneration of particular classes of neurons. Our laboratory focuses on understanding how the physiology of vulnerable neurons contributes to their degeneration as well as studying adaptive physiological responses to degeneration within neural networks.
Our research focuses on Parkinson’s disease (PD) and particularly on the physiological basis of prodromal symptoms of PD (e..g, that appear years before diagnosis). We study it using genetic and other models of PD. We are also interested in the cellular physiology of neurons and circuits in the Basal Ganglia. Our work combines neurophysiological experiments, advanced imaging techniques and mathematical modeling.
The basal ganglia (BG) are a collection of subcortical nuclei that are involved in learning, planning and execution of self-initiated movement, as well as in sensory and affective processing. These nuclei include the striatum, which is the largest input structure of the BG, the globus pallidus, the subthalamic nuclei, the substantial nigra pars reticulata (SNr, one of the output nuclei) and pars compacta (SNc), that is home to the dopamine cells that innervate the striatum. These BG form two feedforward circuits connecting the entire cortex with the frontal cortex: the direct (“go”) and indirect (“stop”) pathways. Half of the spiny projection neurons (SPNs) in the striatum belong to the direct pathway (dSPNs) and the other half to the indirect pathway (iSPNs).
Several movement disorders such as Parkinson’s disease (PD, caused by the loss of SNc dopamine neurons) and Huntington’s disease are characterized by a severe dysfunction of BG circuitry. In PD, as dopamine cells are lost the BG circuitry becomes dysfunctional. In our lab we are interested in understanding the normal physiological function of these circuits and what goes awry in these devastating diseases.
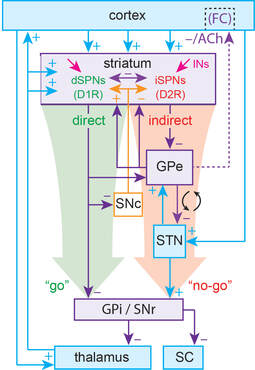
The striatum has the highest expression of markers for dopamine and acetylcholine (ACh) in the brain. It has long been thought that for the proper function of the BG the striatum needs to maintain a so-called balance between these two neuromodulators. In our lab we are interested in the collective dynamics of striatal cholinergic interneurons (CINs) that provide the source of tonic ACh. Cholinergic interneurons (CINs) constitute a tiny fraction of striatal cells, but they are key modulators of striatal circuits and strongly affect SPN excitability and synaptic inputs.
We employ the novel method of microendoscopic calcium imaging, which enables us to record from dozens of genetically defined CINs simultaneously in freely moving mice. Using this cutting-edge technique, we study the collective dynamics of CINs during naturalistic behaviors in healthy intact animals, as well as in parkinsonian mice prior to and following the induction of levodopa-induced dyskinesias.

Microendoscopic calcium imaging of striatal cholinergic interneurons in a freely moving mice
PD is known to result from the death of dopamine (DA) cells in the brain. Understanding why these cells are vulnerable in PD is necessary for the development of neuroprotective therapies. Interestingly, in addition to DA cell death, PD is characterized by the appearance of Lewy pathologies (LPs, intracellular inclusions loaded with a toxic form of the protein alpha-synuclein), not only in DA cells, but also in various other cell types in the brain. These other cells exhibit LPs decades before DA cells do, and well before the onset of the disease, but seem to be less vulnerable in PD than DA cells.
One of the first neuronal populations to exhibit these pathologies are gut innervating neurons in the dorsal motor nucleus of the vagus (DMV). It is widely thought that the appearance of LPs in these neurons accounts for one of the most common prodromal (ie, predating diagnosis) symptoms of PD – severe constipation, but the mechanism for this remains unknown. Interestingly, DMV cell loss is milder than that of DA neurons in the substantia nigra pars compacta, and recent work from our lab has shown that this is likely the result of lower levels of oxidative stress.
Yet, many questions remain unanswered: Why are gut-innervating cells less vulnerable than dopamine cells if they exhibit LPs years earlier? What is the physiological mechanism by which the appearance of LPs in these gut-innervating cells leads to prodromal constipation prior to cell death and what biomarker can be used to identify this pathology? We use slice patch-clamp recordings, juxtacellular in-vivo recordings and histological analysis combined with non-motor behavioral assessment to dissect the physiological mechanisms in DMV neurons under pathological impacts and identify the functional biomarker.
Our work on vagal alpha-synucleinopathy underscores the importance of studying the mechanisms of prodromal symptoms of PD. First, non-motor prodromal symptoms (e.g., constipation, pain) can be severe and require treatment per se. Moreover, once disease modifying therapies for PD are found it will be essential to achieve early detection based on (currently) prodromal symptoms. Thus, we expand our work to other prodromal symptoms.
In particular, the lab is collaborating with several other labs to test our hypothesis that DA neurons in the dorsal raphe nucleus (dRN) are vulnerable in PD (like nigral DA neurons) and that their functional decline leads to prodromal anxiety and depression in PD (AND-PD) by disrupting the dRN efferent pathways. To investigate this, we will combine endoscopic imaging of serotonin release in the dorsal striatum in mouse models of AND-PD with electrophysiological experiments aimed at studying the mechanism of pacemaking in dRN DA neurons and their vulnerability to calcium influx and oxidative stress.

Recent seminal studies have shown that striatal dopamine (DA) release is dissociated from the discharge of nigral DA neurons, and that CINs can activate nicotinic receptors on DA fibers to induce DA release.
We are developing a mathematical model to explain how reciprocal coupling between DA release and CINs can give rise to self-organized spatiotemporal patterns. This model could explain how striatal DA release is independent of the midbrain, and provide a dynamical framework for the realization of the famous striatal “DA-acetylcholine imbalance hypothesis” of neurodegenerative diseases such as PD.


Striatal Neurons Are Recruited Dynamically into Collective Representations of Self-Initiated and Learned Actions in Freely Moving Mice
Lior Tiroshi, Yara Atamna, Naomi Gilin, Noa Berkowitz and Joshua A. Goldberg. eNeuro (2023) https://doi.org/10.1523/ENEURO.0315-23.2023
Featured on eNeuro:
The reliability of neural responses in a given brain region is crucial to its ability to encode information efficiently. Using miniscopes in freely-moving mice, Tiroshi and colleagues monitored individual neurons in the striatum – a brain region central to habitual behaviors and goal-directed learning – across sessions days and weeks apart. They found that while individual striatal neurons respond unreliably during both spontaneous movement and operant conditioning, their collective response remains reliable across sessions. These findings uncover previously unobserved aspects of the collective dynamics of striatal neurons.
Acetylcholine waves and dopamine release in the striatum
Lior Matityahu, Naomi Gilin, Gideon A. Sarpong, Yara Atamna, Lior Tiroshi, Nicolas X. Tritsch, Jeffery R. Wickens & Joshua A. Goldberg. Nature Communications (2023) doi.org/10.1038/s41467-023-42311-5
Non-uniform distribution of dendritic nonlinearities differentially engages thalamostriatal and corticostriatal inputs onto cholinergic interneurons
Osnat Oz, Lior Matityahu, Aviv Mizrahi-Kliger, Alexander Kaplan, Noa Berkowitz, Lior Tiroshi, Hagai Bergman, Joshua A Goldberg. eLife (2022) doi.org/10.7554/eLife.76039
A tonic nicotinic brake controls spike timing in striatal spiny projection neurons
Lior Matityahu, Jeffrey M. Malgady, Meital Schirelman, Yvonne Johansson,
Jennifer Wilking, Gilad Silberberg, Joshua A. Goldberg1 and Joshua L. Plotkin. eLife (2022) doi:10.7554/eLife.75829
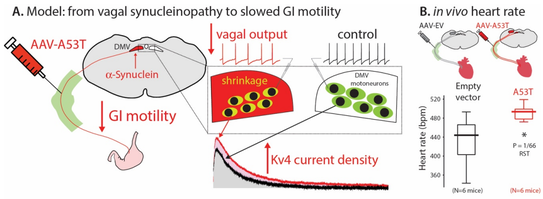
a-Synuclein-induced Kv4 channelopathy in mouse vagal motoneurons drives non-motor parkinsonian symptoms
Chiu W-H, Kovacheva L, Musgrove RE, Arien-Zakay H, Koprich JB, Brotchie JM, Yaka R, Ben-Zvi D, Hanani M, Roeper J, and Goldberg JA*. . Science Advances 7: eabd3994 (2021) doi: 10.1126/sciadv.abd3994
No disease-modifying therapy is currently available for Parkinson’s disease (PD), the second most common neurodegenerative disease. The long nonmotor prodromal phase of PD is a window of opportunity for early detection and intervention. However, we lack the pathophysiological understanding to develop selective biomarkers and interventions. By using a mutant α-synuclein selective-overexpression mouse model of prodromal PD, we identified a cell-autonomous selective Kv4 channelopathy in dorsal motor nucleus of the vagus (DMV) neurons. This functional remodeling of intact DMV neurons leads to impaired pacemaker function in vitro and in vivo, which, in turn, reduces gastrointestinal motility, a common early symptom of prodromal PD. We identify a chain of events from α-synuclein via a biophysical dysfunction of a specific neuronal population to a clinically relevant prodromal symptom. These findings will facilitate the rational design of clinical biomarkers to identify people at risk for developing PD.
Population dynamics and entrainment of basal ganglia pacemakers are shaped by their dendritic arbors
Tiroshi L, Goldberg JA*. PLoS Computational Biology 15(2):e1006782 (2019). doi: 10.1371/journal.pcbi.1006782
The substantia nigra pars reticulata (SNr) is a main output nucleus of the basal ganglia (BG), where inputs from the competing direct and indirect pathways converge onto the same neurons. Interestingly, these inputs are differentially distributed with direct and indirect pathway projections arriving at distal and proximal regions of the dendritic arbor, respectively. We employ a novel method combining theory with electrophysiologi- cal experiments and optogenetics to study the distinct effects of inputs arriving at different locations along the dendrite. Our approach represents a useful compromise between com- plexity and reduction in modelling. Our work addresses the question of high fidelity encoding of inputs by networks of neurons in the new context of pacemaking neurons, which are driven to fire by their intrinsic dynamics rather than by a network state. We provide the first empirical demonstration that dendritic delays can introduce latencies in the responses of a population of neurons that are commensurate with synaptic delays, sug- gesting a new role for SNr dendrites with implications for BG function.
Mutant α-Synuclein overexpression induces stressless pacemaking in vagal motoneurons at risk in Parkinson’s disease
Lasser-Katz E†, Simchovitz A†, Chiu W-H†, Oertel WH, Sharon R, Soreq H, Roeper J, Goldberg JA*. Journal of Neuroscience 37(1):47-57 (2017). †Equal contribution
Overexpression of mutant alpha-synuclein causes Parkinson’s disease, presumably by driving neurodegeneration in vulnerable neu- ronal target populations. However, the extent of alpha-synuclein pathology (e.g., Lewy bodies) is not directly related to the degree of neurodegeneration across various vulnerable neuronal populations. Here, we show that, in contrast to dopamine neurons in the substantia nigra, vagal motoneurons do not enhance their excitability and oxidative load in response to chronic mutant alpha-synuclein overexpression. Rather, by downregulating their voltage-activated calcium channels, vagal motoneurons acquire a stressless form of pacemaking that diminishes mitochondrial and cytosolic oxidative stress. Emulating this endogenous adaptive response to alpha-synuclein overexpression could lead to novel strategies to protect dopamine neurons and perhaps delay the onset of Parkinson’s disease.
Selective remodeling of glutamatergic transmission to striatal cholinergic interneurons after dopamine depletion
Aceves Buendia J de J, Tiroshi L, Chiu W-H, Goldberg JA*. . European Journal of Neuroscience 49:824-833 (2019) doi: 10.1111/ejn.13715.
The widely held view that the pathophysiology of Parkinson’s disease arises from an under-activation of the direct pathway striatal spiny neurons (dSPNs) has gained support from a recently described weakening of the glutamatergic projection from the parafas- cicular nucleus (PfN) to dSPNs in experimental parkinsonism. However, the impact of the remodeling of the thalamostriatal pro- jection cannot be fully appreciated without considering its impact on cholinergic interneurons (ChIs) that themselves preferentially activate indirect pathway spiny neurons (iSPNs). To study this thalamostriatal projection, we virally transfected with Cre-depen- dent channelrhodopsin-2 (ChR2) the PfN of Vglut2-Cre mice that were dopamine-depleted with 6-hydroxydopamine (6-OHDA). In parallel, we studied the corticostriatal projection to ChIs in 6-OHDA-treated transgenic mice expressing ChR2 under the Thy1 pro- moter. We found the 6-OHDA lesions failed to affect short-term synaptic plasticity or the size of unitary responses evoked optoge- netically in either of these projections. However, we found that NMDA-to-AMPA ratios at PfN synapses—that were significantly larger than NMDA-to-AMPA ratios at cortical synapses—were reduced by 6-OHDA treatment, thereby impairing synaptic integra- tion at PfN synapses onto ChIs. Finally, we found that application of an agonist of the D5 dopamine receptors on ChIs potentiated NMDA currents without affecting AMPA currents or short-term plasticity selectively at PfN synapses. We propose that dopamine depletion leads to an effective de-potentiation of NMDA currents at PfN synapses onto ChIs which degrades synaptic integration. This selective remodeling of NMDA currents at PfN synapses may counter the selective weakening of PfN synapses onto dSPNs in parkinsonism.
Activity patterns in the neuropil of striatal cholinergic interneurons in freely moving mice represent their collective spiking dynamics
Rehani R†, Atamna Y†, Tiroshi L†, Chiu W-H, Aceves Buendia JJ, Martins GJ, Jacobson GA, Goldberg JA*. . eNeuro 6(1):e0351-18.2018 1-16. (2019) doi: 10.1523/ENEURO.0351-18.2018. †Equal contribution
Cholinergic interneurons (CINs) are key modulators of the striatal microcircuitry that are necessary for assigning action value and behavioral flexibility. We present a first endoscopic imaging study of multiple molecularly identified CINs in freely moving mice. We reveal the presence of activity patterns in the CIN neuropil. We then use ex vivo electrophysiological and imaging techniques to show that the neuropil signal is the integrated fluorescence arising from the axodendritic arbors of CINs dispersed throughout the striatum. We conclude that the neuropil signal acts as a mean-field readout of the striatal CIN network activity.

PhD student
Dynamics of mono-amines release effects on the striatum

MSc Student
dendritic nonlinearities in striatal cholinergic interneurons

MSc Student
the biophysiological properties of Dopamine neurons within the midbrain that contribute non-motor-symptoms to prodromal Parkinson’s Disease.

Undergraduate Student
The effects of Alpha-synuclein injection to the dorsal raphe nucleus on the Mitochondrial oxidative stress levels of dopaminergic cells.
Dr. Gilad Jacobson
Dr. Efrat Lasser-Katz
Dr. Jose Aceves-Buendia
Dr. Wei-Hua Chiu
Dr. Ruth Musgrove
Lior Tiroshi (PhD, now at Northwestern University)
Rotem Rehani (MSc)
Meital Schirelman (MSc)
We are currently seeking excellent and highly motivated graduate students/post-docs to join the lab. Candidates will learn to conduct bleeding edge imaging and electrophysiological experiments. There are multiple projects to join.



First lab outing since Covid-19. We had much to celebrate (right to left): Lior Tiroshi is on her way to her postdoc with DanielDombeck; Lior M published his first preprint on biorxiv; Nadine and Osnat are completing their MSc; Yara finished Medical School (!!!) & I was tenured a while back.
Website designed by toornet
My interest in the brain began in earnest when, as an officer in the Israeli military, I read Steven Pinker’s book The Language Instinct. I was intrigued by the fact that there exist dedicated brain circuits that evolved to give rise to a universal human grammar. So, during my undergraduate studies in Physics, I took a year-long course and seminar with the Late Prof. Edit Doron on Chomskyan Generative Grammar which at the time was called Government and Binding Theory. Thus, when I enrolled in the ICNC graduate program in Computational and Systems Neuroscience, I had my mind set on using tools in physics and dynamical systems theory to model how a human listener can decode syntax on the fly.
However, as I began to study about the brain and its circuits, I realized that just as human language has an intricate and interesting structure so does the brain itself. Thus, slowly but surely I switched my attention and interest to the question of how the collective dynamics of neurons arises in neural networks. I was fascinated by the presence of phase transitions or bifurcations in the dynamical activity of brain circuits. In particular, the onset of pathological oscillations and excessive neuronal synchrony in the cortico-basal ganglia loops became the focus of my work with Hagai Bergman and David Hansel here at the Hebrew University and with Dieter Jaeger (Emory University). I also studied the dynamics of ongoing activity in the primary visual cortex with Haim Sompolinsky.
After acquiring a PhD in systems level neurophysiology, I became interested in better understanding the cellular physiology of basal ganglia neurons. Therefore, I spent my postdoctoral training with Charlie Wilson (University of Texas at San Antonio) studying the intrinsic properties and calcium dynamics of striatal cholinergic interneurons (CINs).
After my post-doctoral fellowship, and before making a life-long commitment to academic science, I became curious about translational neuroscience. Therefore, I took a position at a medical device company (BioControl Medical Ltd.), where I used my combined training in physics and neurophysiology to head the development of a cuff electrode for vagal nerve stimulation. While the industry experience provided me with a deeper understanding of the translational aspect of neuroscience research, and strengthened my commitment to keeping a clinical angle to my own research, it also convinced me to pursue a career in academia. Thus, in 2009, I accepted a faculty position of research assistant professor at Northwestern University to work with Jim Surmeier. My goal was to extend my knowledge to molecular biological techniques and two-photon laser scanning microscopy (2PLSM).
As an independent PI (since 2013), my lab continues to study the pathophysiology of PD, meaning how brain circuits and brain activity are altered in models of PD. This work is typically relevant to understanding late stage PD. In recent years, I have switched my focus to the pathophysiology of prodromal PD (meaning, the very early stages of the disease process that precede diagnosis by up to 20 years). Here we study cell physiological underpinnings of selective vulnerability in PD models. In particular, we study how Lewy pathologies and alpha-synuclein aggregation (that spread throughout the brain stem during prodromal PD) give rise to the various non-motor symptoms (NMS) of PD. The great promise of this approach is that if we can identify physiological changes that are unique to and driven by these early pathological processes, we may be able to diagnose PD – and provide therapies – earlier.
2019-Today: “Pirhai-Mehkar”, a direct PhD program at neurobiology.
2016-2019: Psycho-biology student, the Hebrew University.
Today my research focuses on cellular physiology combined with two-photon-laser-scanning-microscopy (2PLSM) to better understand striatal cholinergic interneurons (CIN) at the Striatum.
I finished my BSc in biomedical sciences in the Hebrew University, and continued for an MSc in the lab. My research focuses on dendritic nonlinearities in striatal cholinergic interneurons ,and in particular the distribution of NaP and HCN channels along the dendritic arbor using electrophysiological investigation combined with optogenetics.
I grew up in Jatt village. My BSc was in biochemistry and food science at the Hebrew University of Jerusalem. When I finished my BSc, I became interested in neurobiological research and I was fortunate to join Prof. Goldberg’s lab.
We hope that with a better understanding of the early underlying processes of the disease, we can help identify druggable targets for early neuroprotective interventions that may be able to prevent or alter the course of the disease.
Born and raised in Alon Shvut, Gush Etzion. Currently in the second year of my undergraduate degree in biomedical sciences.