
Senior lecturer
We are interested in studying how the brain encodes cognition at the brain-wide level. Sensory integration, perception, memory and learning are just several examples of the cognitive functions that we aim to understand. In the lab we take a mesoscale approach in which we aim to simultaneously image as many brain areas as possible as mice perform complex behavioral tasks. Complementing the mesoscale approach, we implement a microscale approach, using multi-area two-photon microscopy, optogenetics and labeling techniques to dissect the relevant neuronal sub-populations. We further study more natural behavior such as social interactions in both the healthy and diseased brain. Our goal is to obtain a cognitive brain-wide map where relevant information flows through specific processing streams based on demand.”
The brain is responsible for thinking, combining mental processes such as sensory integration, perception and working memory, grouped together under one broad definition: cognition. The holy grail of neuroscience is to understand how the brain encodes cognition, unifying many specific processes into a coherent intellect. The biggest obstacle in achieving this challenge is the complex nature of the brain, an extremely complicated organ containing billions of neurons entangled together forming a dynamic, ever-changing network. Even more overwhelming is the plastic and dynamic nature of the brain, constantly changing and forming new synapses, knowledge and experience. There are no two similar brains on earth, and even the same brain can give different outcomes to seemingly identical incoming stimuli. Neuronal populations are multidimensional, merged in an eternal, ever-changing loop between the external and internal worlds.
In the lab, we are interested in studying how the brain encodes cognition at the brain-wide level. We adopt a mesoscale approach in which we aim to simultaneously image as many brain areas as possible as mice perform complex behavioral tasks involving different cognitive functions. Complementing the mesoscale approach, we use multi-area two-photon microscopy, optogenetics and labeling techniques to dissect the relevant neuronal sub-populations responsible for different cognitive functions. Our goal is to obtain a cognitive brain-wide map that will aid in understanding cognition as a whole in both the healthy and diseased brain.
An example study is finding the cortical location of short-term memory. To address this question, we trained mice on a memory task and simultaneously imaged the whole dorsal cortex. We found very surprising results in which short-term memory was located at very different locations based on the internal strategy of the mouse (See Figure). Thus, cognitive processing is flexible and dynamic requiring us to use wide-field and flexible approaches in order to obtain a comprehensive understanding.
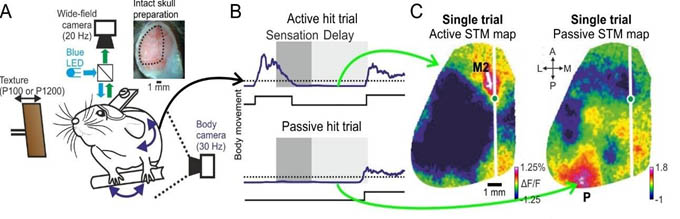
Figure legend: A) Mice were trained on a whisker dependent go/no-go task with a short-term memory (STM) component, while imaging the whole dorsal cortex and monitoring body movements.B) Body movement vector in two example Hit trials, one active and the other passive during the sensation period. C) STM maps in a single active or passive trial, showing a dissociation of frontal M2 and posterior P respectively.
One of our main goals is to understand cognition as a whole rather than focusing on a specific aspect of cognition. To do this we aim to train mice on different behavioral tasks, each highlighting a specific cognitive function. Example tasks are go/no discrimination with delayed response, delayed match-to-sample task and a two-alternative force choice task.
We use wide-field imaging to simultaneously image large parts of the cortex. This can be done for either one or two cortical hemispheres. We can image calcium dynamics from up to 31 different cortical areas as mice perform a complex task. In addition, we aim to image subcortical areas using a multi-fiber approach (Sych et al. 2019). A combination of both cortical wide-field imaging and multi-fiber photometry in one mouse can enable us to maximize our observation across the brain. Using transgenic mice, we can target different neuronal subtypes with a calcium indicator, for example different inhibitory subtypes or different cortical layers. Thus, we are able to compare the brain-wide activity of different neuronal subtypes to unravel the global mechanism of different cognitive functions.
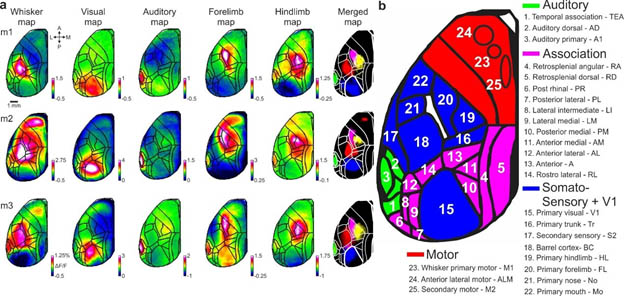
Figure legend: Cortical functional mapping and area definitions a, Mapping session examples including five stimulus modalities: whisker, visual, auditory, forelimb and hindlimb (Methods). Mean activation maps are shown for each stimulus type for two example mice. Color denotes normalized fluorescence (ΔF/F). b, Full names and abbreviations of 25 areas. Areas were divided into auditory (green), association (pink), somatosensory and primary visual cortex (blue) and motor (red) cortices.
Complementing the mesoscale approach, we will use a microscale approach to target specific areas of interests. We use a state-of-the-art multi-area two-photon microscope to image single cells in multiple non-contiguous areas. We can also label different cells based on their projection output and thus start segregating neuronal population into different sub-populations. To test causality, we use optogenetic manipulation to perturb specific neuronal sub-population as mice perform the task.
Social interaction is a key function for our daily life, and aberrant in many disorders such as autism and schizophrenia. We use a novel method of chronic implant – an optical fiber array, directed to many brain areas simultaneously, that enables us to study the brain network in freely moving mice. We aim to study the brain-wide social and cognitive networks in mouse model of schizophrenia, autism and alzheimer’s disease.
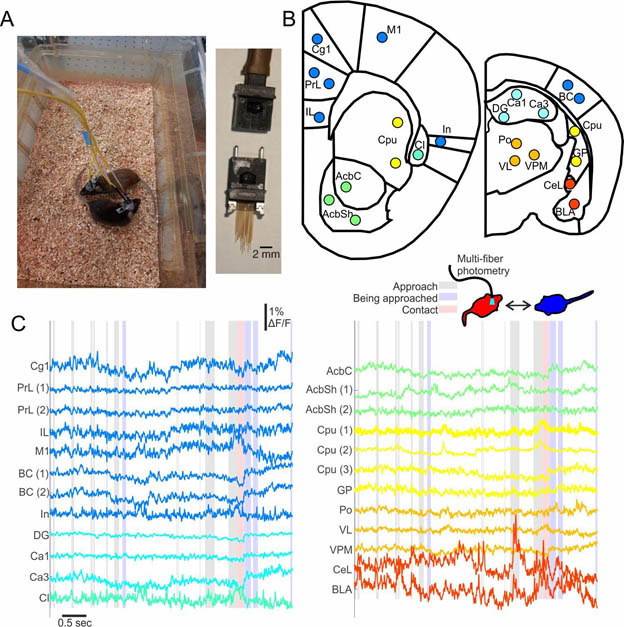
Figure legend. Multi-fiber photometry during social interactions. A) Multi-fiber recording from two freely moving mice (left) and the multi-fiber implant (right). B) 24 fiber target locations. C) Activity in 24 recording sites during social interaction between two mice (recorded red vs. blue). Shaded epochs depict an approach (gray), being approached (blue) or contact (red) of the red mouse in relation to the blue mouse. Abbreviations – in cortex – cingulate (Cg), prelimbic (PrL), infralimbic (IL), insular (In), motor primary (M1), barrel (BC). In hippocampus – Ca1, Ca3, and dentate gyrus (DG). In basal forebrain (with nearby additions) – Caudate putamen (Cpu), globus pallidus (GP), claustrum (Cl), nucleus accumbens shell (AcbSh) and core (AcbC). In thalamus – ventrolateral (VL), ventral posterior medial (VPM) and posterior medial (Po). In amygdala – central (CeL) and basolateral (BLA).
Marmor, O., Pollak, Y., Doron, C., Helmchen, F., & Gilad, A. (2023). History information emerges in the cortex during learning. eLife.
To study the relationship between history and learning, we continuously imaged cortex-wide calcium dynamics as mice learn to use their whiskers to discriminate between two different textures. We mainly focused on comparing the same trial type with different trial history, that is, a different preceding trial. We found trial history information in barrel cortex (BC) during stimulus presentation. Importantly, trial history in BC emerged only as the mouse learned the task. Next, we also found learning-dependent trial history information in rostrolateral (RL) association cortex that emerges before stimulus presentation, preceding activity in BC. Trial history was also encoded in other cortical areas and was not related to differences in body movements. Interestingly, a binary classifier could discriminate trial history at the single trial level just as well as current information both in BC and RL. These findings suggest that past experience emerges in the cortex around the time of learning, starting from higher-order association area RL and propagating down (i.e., top-down projection) to lower-order BC where it can be integrated with incoming sensory information. This integration between the past and present may facilitate learning.
Gallero-Salas, Y., Han, S., Sych, Y., Voigt, F. F., Laurenczy, B., Gilad, A.* & Helmchen, F.*, (2020). Sensory and Behavioral Components of Neocortical Signal Flow in Discrimination Tasks with Short-Term Memory. Neuron 109, 1–14. * – co-senior.
Here, we implemented a demanding two-step approach to train mice on both a texture and an auditory memory task. We found that sensory processing is encoded in different association areas, but short-term memory is located in M2 or P based on the behavioral strategy rather than stimulus modality. These results indicate that processing streams may be dependent on internal states rather than external.
Gilad, A., Maor, I., & Mizrahi, A. (2020). Learning-related population dynamics in the auditory thalamus. eLife, 9, 1–18.
In this paper, using fiber photometry, we imaged population responses in the auditory thalamus in a trial-by-trial manner as mice learned to discriminate between two sounds. We found rich learning-related activity implying that thalamus encodes cognition. This study adds to our understanding that cognition is encoded in a brain-wide manner.
Gilad, A., & Helmchen, F. (2020). Spatiotemporal refinement of signal flow through association cortex during learning. Nature Communications, 11(1), 1–14.
Here, we imaged twenty-five cortical areas in a trial-by-trial manner as mice learned to discriminate between two textures. We showed learning-related dynamics all over the cortex, including in several surprising areas, even way prior to learning.
Gilad, A., Gallero-Salas, Y., Groos, D., and Helmchen, F., (2018). Behavioral strategy determines frontal or posterior location of short-term memory in neocortex. Neuron 99, 814–828.e7.
This is the first study to use wide-field imaging in mice during a complex memory task. We showed for the first time that WM during the same task is located in distinctly different areas, either in frontal M2 or posterior P area. Area P is a newly identified area and outlines different processing streams. In addition, we applied optogenetics and 2P imaging in a zoom-in second step of the same mouse.
Gilad, A., and Slovin, H. (2015). Population responses in V1 encode different figures by response amplitude. Journal of Neuroscience. 35, 6335–6349.
This is a critical paper showing that labelling different figures in the visual field is encoded by a V1 population amplitude code. This paper adds to our hypothesis that cognitive functions are encoded at the population level.
Gilad, A., and Slovin, H. (2015). Population responses in V1 encode different figures by response amplitude. Journal of Neuroscience. 35, 6335–6349.
This is a critical paper showing that labelling different figures in the visual field is encoded by a V1 population amplitude code. This paper adds to our hypothesis that cognitive functions are encoded at the population level.

Postdoctoral fellow
PhD candidate

PhD candidate

MD/PhD candidate

MSc student

MSc student

MSc student

MSc student

Computational MD candidate

Computational MD candidate

MD candidate

MD candidate
MD candidate

Undergraduate student

Undergraduate student

Undergraduate student

Undergraduate student

Undergraduate student

Undergraduate student

Undergraduate student

Undergraduate student

Undergraduate student
Genotyping specialist
Chen Alkobi (MD candidate)
Arwa Massalha (Undergraduate student)
Yuval Shlomo (Undergraduate student)
Zehava Berenstein (Undergraduate student)
Netanel Corem (Undergraduate student)
Dor Bilenko (Undergraduate student)
Mona Melhem (Undergraduate student)
Nir Butbul (Undergraduate student)
Eitan Bergman (Undergraduate student)
Magal Gafni (Undergraduate student)
Shachar Springer (Undergraduate student)
Michael Cynovich (Undergraduate student)
Natalie Kerniacob (Undergraduate student)
Peer Hadar (Undergraduate student)
Ariel Biton (Undergraduate student)
Elnatan Bitensky (Undergraduate student)
Eitan Navon (Undergraduate student)
Mohamed Abd al-Rahm (Undergraduate student)
Lama Naamneh (Undergraduate student)
Mor Vazana (Undergraduate student)
Or Naim (Undergraduate student)
We are looking for highly motivated postdoc and student to join the lab.
Website designed by toornet
I earned my B.Sc. from the Hebrew University of Jerusalem majoring in life sciences and mathematics. I obtained my PhD from Bar-Ilan University under the supervision of Prof. Hamutal Slovin where I studied perceptual grouping mechanisms in the primary visual cortex of behaving monkeys. I continued for postdoctoral training at the University of Zurich at Prof. Fritjof Helmchen’s lab where I studied brain-wide dynamics of different cognitive function in the mouse model. I returned to Israel for a one-year Marie-Curie return phase and studied the auditory thalamus during learning. In 2019, I opened an independent research lab at the Faculty of Medicine at the Hebrew University. Our research focuses on brain-wide dynamics of cognition in the mouse model.