
Associate Professor
Almost all our actions are driven by motivation. Normally, motivation is the driving force behind adaptive behaviors. However, losing control over motivated behavior is detrimental and leads to one of the most devastating behavioral disorders – addiction. This may be true not only for drugs of abuse but for natural rewards like palatable food as well.
Our lab is interested in understanding the neurobiological processes that turn normal motivated behavior into an addiction. We investigate this by combining state-of-the-art tools with behavioral models of pathological motivation to target and manipulate specific circuits in the reward system and evaluate synaptic plasticity in drug addiction and overeating.
Behavior is the result of concerted action of neurons from various brain regions. Each brain region may contain many types of neurons, each expressing different genes and projecting to different brain regions. Thus, not all neurons in a brain region may be relevant to a specific behavior. In fact, neighboring neurons may be involved in completely different, sometimes opposing, behaviors depending on their connectivity with neurons in other regions. Therefore, in order to truly understand the neural substrates of a specific behavior it is essential to identify and target specific neurons with known connectivity patterns.
The nucleus accumbens (NAc) and the ventral pallidum (VP) are two regions in the basal ganglia that are deeply involved in the execution of motivated behavior, and uncontrolled motivated behavior is tightly linked with permanent synaptic changes in these two regions. The NAc and the VP receive various inputs and project to many brain regions and thus neurons of the NAc and VP participate in many different neural circuits. Moreover, the NAc and the VP are themselves interconnected in a complex manner. Recent studies show that synaptic plasticity in the NAc after exposure to drugs is not homogenous and occurs at specific synapses. Thus, loss of control on motivated behavior is likely to be mediated by subpopulations in the NAc and the VP that form dedicated microcircuits. Identifying and targeting these specific microcircuits and the synaptic changes therein leading to addictive behavior is therefore crucial.
The first step in understanding how the brain produces motivational or addictive behavior is to understand the exact infrastructure of the reward system. Which neurons are connected to which? How do specific regions in the reward system affect the activity of other regions in the system? One of our goals is to dissect cell-type-specific circuits in the reward system and examine the physiology of specific cells in those circuits. We are specifically interested in the ventral pallidum and the nucleus accumbens and how specific neurons in those regions interact with each other and with other regions of the reward system. To achieve this we utilize advanced neural tracing tools combined with in vivo and ex vivo optogenetics, chemogenetics and electrophysiology in various lines of transgenic mice.
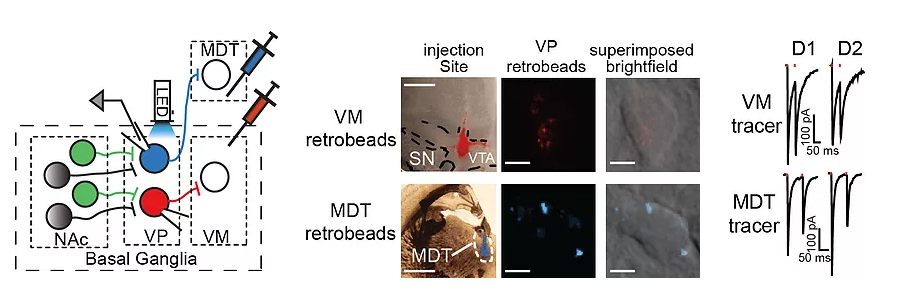
By injecting Cre-ChR2 into the nucleus accumbens (NAc) and retrobeads into the thalamus (MDT) or ventral mesencephalon (VM) of D1-Cre or D2-Cre mice we discovered that D1-MSNs contact neurons in the VP that project to the VM and thus are part of the indirect pathway.
(Adapted from Kupchik et al. 2015 Nature Neuroscience)
What happens in the brain that turns normal motivation into an addiction? Addiction is believed to be underlied by alterations of specific synapses in the reward system. These alterations may cause the uncontrollable desire to consume the drug. Which synapses are altered during the development of addiction? What exactly are the changes? Can we attenuate addictive behavior by compensating for the synaptic alterations caused by drugs? A main goal in our lab is to reveal the permanent synaptic changes that occur in drug addiction in specific neurons of the reward system. We then aim to target the altered synapses with the intention to attenuate addictive behavior. To achieve this we use animal models of drug addiction together with electrophysiological and optogenetic/chemogenetic tools in transgenic mice.
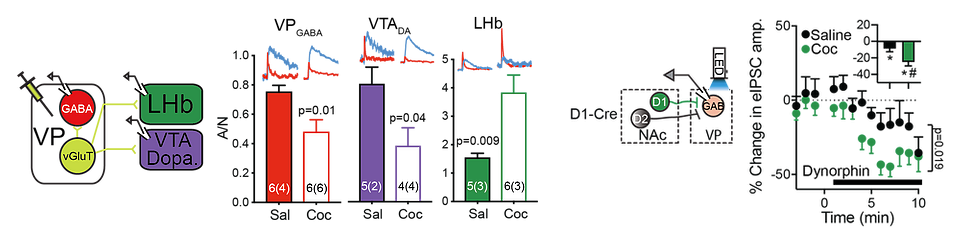
Abstinence from repeated cocaine injections alters the glutamatergic output of ventral pallidal neurons, measured as AMPA/NMDA (A/N), to different targets (left) and potentiates the inhibitory effect of dynorphin on GABA release from nucleus accumbens terminals in the ventral pallidum expressing the D1 dopamine receptor.
(Adapted from Inbar et al. and Levi et al. 2020 J. Neurosci.)
Obesity is among the most devastating health problems in modern society. It is mainly attributed to the sedentary modern lifestyle and to the high caloric content of processed foods, but the increasing palatability of food cannot be ignored. Do obese people overconsume calorie-rich food because of its rewarding aspects? If so, could obesity be underlied, at least in part, by changes in the reward system that resemble those occurring in pathological conditions of addiction? In the lab we strive to understand whether the reward system is involved in the development of obesity. We use an animal model of obesity and examine the reward pathway with electrophysiological and optogenetic/chemogenetic tools.
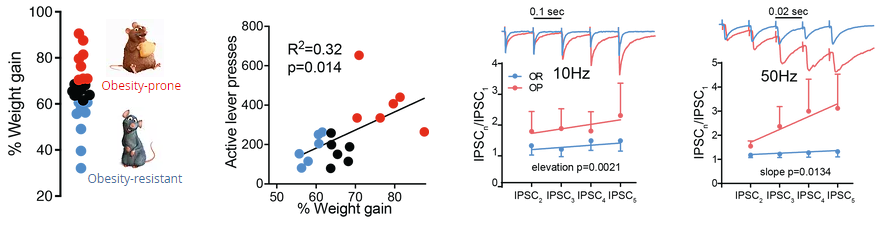
Animals that gained the most weight in a diet-induced obesity paradigm (obesity-prone, red) also showed higher motivation to obtain palatable food comapred to obesity-resistant animals (blue) and a short-term potentiation of the GABAergic input to the ventral pallidum.
(Adapted from Inbar*, Gendelis*, et al. 2019 Addiction Biology and Gendelis*, Inbar* et al. 2020 J. Neurosci.)
Israel Science Foundation (ISF)
The Milton Rosenbaum Endowment fund
The National Institute for Psychobiology in Israel (NIPI)
The United States – Israel Binational Science Fund (BSF)
The Israeli Authority on Drug Abuse
The Abisch-Frenkel Foundation for the Promotion of Life Sciences
Levi, L. A., Inbar, K., Tseiger, E., & Kupchik, Y. M. (2025). A ventral pallidal glutamatergic aversive network encodes abstinence from and reexposure to cocaine. Science Advances, 11(30), eadu6074.
DOI: https://doi.org/10.1126/sciadv.adu6074
Bernat, N., Campbell, R., Nam, H., Basu, M., Odesser, T., Elyasaf, G., … & Kupchik, Y. M. (2024). Multimodal interrogation of ventral pallidum projections reveals projection-specific signatures and effects on cocaine reward. Journal of Neuroscience.
Inbar K, Levi LA, Bernat N, Odesser T, Inbar D, Kupchik YM. Cocaine Dysregulates Dynorphin Modulation of Inhibitory Neurotransmission in the Ventral Pallidum in a Cell-Type-Specific Manner. J Neurosci. 2020;40(6):1321-1331. doi:10.1523/JNEUROSCI.1262-19.2019
This paper shows the changes in dynorphin functions in the ventral pallidum after cocaine. We show that the suppressing effect of dynorphin on GABA release in the ventral pallidum is potentiated when GABA is released on GABA neurons but weakened when GABA is released on glutamate neurons.
Levi LA, Inbar K, Nachshon N, et al. Projection-Specific Potentiation of Ventral Pallidal Glutamatergic Outputs after Abstinence from Cocaine [published correction appears in J Neurosci. 2020 Apr 29;:]. J Neurosci. 2020;40(6):1276-1285. doi:10.1523/JNEUROSCI.0929-19.2019
Here we show that different ventral pallidal glutamatergic projection neurons make synapses of different characteristics with their different targets and that abstinence from cocaine potentiates the synapses of VP glutamatergic neurons with aversion-related targets but depresses the synapses with reward-related targets.
Inbar D, Gendelis S, Mesner S, Menahem S, Kupchik YM. Chronic calorie-dense diet drives differences in motivated food seeking between obesity-prone and resistant mice. Addict Biol. 2020 May;25(3):e12753. doi: 10.1111/adb.12753. Epub 2019 Apr 22. PMID: 31012232.
This paper shows that mice that gain the most weight when fed chronically with high-fat-high-sugar diet (obesity-prone mice) show stronger motivation to obtain palatable food compared to mice that gained the least weight (obesity-resistant mice) not only after the diet but also before it.
Gendelis S, Inbar D, Inbar K, Mesner S, Kupchik YM. Metaplasticity in the Ventral Pallidum as a Potential Marker for the Propensity to Gain Weight in Chronic High-Calorie Diet. J Neurosci. 2020 Dec 9;40(50):9725-9735. doi: 10.1523/JNEUROSCI.1809-20.2020. Epub 2020 Nov 16. PMID: 33199503; PMCID: PMC7726535.
Here we show that mice that gain the most weight when fed chronically with high-fat-high-sugar diet (obesity-prone mice) show substantial physiological differences in ventral pallidal cells compared to mice that gained the least weight (obesity-resistant mice). Some of these differences were present before starting the diet.

PhD Student
The involvement of the ventral pallidum in diet-induced obesity.

PhD Student
The role of VP-projecting NAc D1-MSNs in withdrawal from cocaine

MD/PhD Student
Characterization of the glutamatergic neurons of the ventral pallidum and their involvement in cocaine addiction.

MD/PhD Student
Extent of involvement of different ventral pallidum projection neurons in drug-seeking behavior, and their anatomical and physiological characterization.

MD/PhD Student
The influence of bariatric surgery on the reward system

Lab manager

BSc Student
Alumni:
Noa Nachshon (2019) – Research Assistant
Tal Shapira (2019) – BSc student
Ava Kamoun (2019) – BSc student
Barak Zlakishvili (2018) – BSc student
Shanee Mesner (2018) – BSc student
Sivane Brodache (2017) – BSc student
Nofar Baruch (2017) – Research Assistant
Shira Menachem (2016) – BSc student
Lama Abed Al Razaq (2016) – BSc student
Esther Goldlist (2015) – BSc student
My interest in the connection between neurobiology and behavior began during my studies for the BSc degree in Psychobiology at the Hebrew University. After receiving my BSc I joined the laboratory of the late Prof. Itzchak Parnas at the Hebrew University and studied, for 7 years, the mechanisms controlling the time course of synaptic release in neuromuscular junctions. I discovered that while calcium is essential for release to occur, the time course of synaptic release is determined by voltage-dependent presynaptic G-protein coupled receptors that modulate the activity of the release machinery.
With my expertise in synaptic physiology, I joined for my postdoc the laboratory of Dr. Peter Kalivas at the Medical University of South Carolina to study synaptic alterations driven by withdrawal from drugs of abuse or from chronic highly-palatable food. In my postdoc I investigated two central regions of the reward system – the nucleus accumbens (NAc) and the ventral pallidum (VP) – and have described substantial changes they undergo after withdrawal. I also revealed that these two regions are interconnected in a more sophisticated pattern than previously thought.
In September 2014 I was recruited as a Senior Lecturer by the department of Medical Neurobiology in the Faculty of Medicine of the Hebrew University. In my laboratory we continue to investigate the synaptic and circuit underpinnings of drug reward and abstinence and of diet-induced obesity using advanced circuit dissection and cellular-specific tools.
Apart from “work”, I play basketball, play the guitar and play with my 3 kids. I am also a (Jewish) genealogy enthusiast and am leading several global projects directed at making genealogical information available on the internet. My expertise is Jews from Ukraine, and particularly those that moved from eastern Europe to South America.