
Assistant Professor
Our lab studies basic principles of brain operation, with special emphasis on the role of synaptic connectivity on local and global brain function. We apply two main approaches in our investigations. (1) Examining the foundations of basic brain functions using a simple system, the compact brain of the roundworm C. elegans. In this way we can trace complex questions about the brain to their roots. (2) Genetically synthesizing new synaptic connections in C. elegans neural circuits, with the aim of ultimately engineering entire brain circuits. This synthetic biology approach, applied to a whole animal, provides deep insights into the causative links between neuronal connectivity and brain function.
We study the relations between the structure of brain neural circuits and the behaviors that they produce, and in particular, how changes in neural circuit connectivity and signaling lead to behavioral modifications. To this end, we focus on the relatively simple nervous system of the nematode worm C. elegans. Our lab investigates how the C. elegans nervous system responds to sensory loss and to neuronal damage, providing insights about the molecular and cellular processes of adaptation and recovery. We also examine normal healthy processes of experience-dependent learning and its neuronal underpinnings. In addition to natural neural circuit modifications, we also generate synthetic changes, by genetically inserting new synaptic connections between target neurons, creating new artificial signaling pathways within the nervous system. In this way we can circumvent the effects of neuronal damage, design new behaviors, and attain a fuller causal understanding of structure-function relations in the nervous system.
We have previously shown that loss of touch sensation in C. elegans leads to adaptive changes in behavior, including enhanced odor-locomotion coupling. The goal of the current project is to unravel the role of the touch neurons in producing these behavioral adjustments and how they signal to the rest of the system that touch information is unavailable.
Cross-modal signaling between touch and smell enables behavioral adjustment to sensory loss
Although the nervous system appears to have a lateral symmetrical structure, there are many asymmetries to be found between its left and right sides. The goal of this project is to reveal lateral asymmetry in synaptic connectivity, analyze the prevalence and consistency of such asymmetries and unravel their functional implications (e.g., parallel processing, signal integration). The project is funded by an Israeli Science Foundation grant.

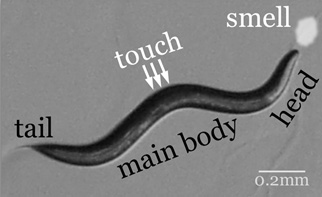
The left and right touch receptor neurons, ALML and ALMR.
A prominent feature of the nervous system is its ability to integrate new information, translated into relevant behavioral changes through synaptic and neuronal plasticity. The goal of this project is to study molecular processes within specific sensory neurons that accompany such learning and plasticity, and to unravel how more complex forms of learning take place.
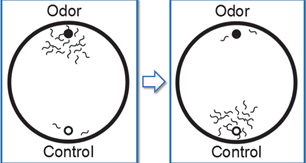
Worms normally attracted to an odor can be trained to avoid it by associating the odor with a negative stimulus.
Israel Science Foundation
Cohen, N., & Rabinowitch, I. (2024). Resolving transitions between distinct phases of memory consolidation at high resolution in Caenorhabditis elegans. iScience, 27(11).
https://doi.org/10.1016/j.isci.2024.111147
Memory consolidation following learning is a dynamic and complex process comprising several transitions between distinct memory phases. Although memory consolidation has been studied extensively, it remains difficult to draw an integral description that can delimit the transition points between specific memory phases at the behavioral, neuronal, and genetic levels. To this end, we have developed a rapid and robust aversive conditioning protocol for the nematode worm Caenorhabditis elegans, tracing memory consolidation within the first hour post conditioning and then up to 18 h post conditioning. This made it possible to uncover time-dependent involvement of primary sensory neurons, transcription and translation processes, and diverse gene populations in memory consolidation. The change in neuronal valence was strong enough to induce second order conditioning, and was amenable to considerable modulation in specific mutant strains. Together, our work lends memory consolidation to detailed temporal and spatial analysis, advancing system-wide understanding of learning and memory.
Birari, V. S., & Rabinowitch, I. (2024). Asymmetry in synaptic connectivity balances redundancy and reachability in the Caenorhabditis elegans connectome. iScience, 27(9).
https://doi.org/10.1016/j.isci.2024.110713
The brain is overall bilaterally symmetrical, but also exhibits considerable asymmetry. While symmetry may endow neural networks with robustness and resilience, asymmetry may enable parallel information processing and functional specialization. How is this tradeoff between symmetrical and asymmetrical brain architecture balanced? To address this, we focused on the Caenorhabditis elegans connectome, comprising 99 classes of bilaterally symmetrical neuron pairs. We found symmetry in the number of synaptic partners between neuron class members, but pronounced asymmetry in the identity of these synapses. We applied graph theoretical metrics for evaluating Redundancy, the selective reinforcement of specific neural paths by multiple alternative synaptic connections, and Reachability, the extent and diversity of synaptic connectivity of each neuron class. We found Redundancy and Reachability to be stochastically tunable by the level of network asymmetry, driving the C. elegans connectome to favor Redundancy over Reachability. These results elucidate fundamental relations between lateralized neural connectivity and function.
Staum, M., Abraham, A. C., Arbid, R., Birari, V. S., Dominitz, M., & Rabinowitch, I. (2024). Behavioral adjustment of C. elegans to mechanosensory loss requires intact mechanosensory neurons. PLoS biology, 22(7), e3002729. https://doi.org/10.1371/journal.pbio.3002729
Sensory neurons specialize in detecting and signaling the presence of diverse environmental stimuli. Neuronal injury or disease may undermine such signaling, diminishing the availability of crucial information. Can animals distinguish between a stimulus not being present and the inability to sense that stimulus in the first place? To address this question, we studied Caenorhabditis elegans nematode worms that lack gentle body touch sensation due to genetic mechanoreceptor dysfunction. We previously showed that worms can compensate for the loss of touch by enhancing their sense of smell, via an FLP-20 neuropeptide pathway. Here, we find that touch-deficient worms exhibit, in addition to sensory compensation, also cautious-like behavior, as if preemptively avoiding potential undetectable hazards. Intriguingly, these behavioral adjustments are abolished when the touch neurons are removed, suggesting that touch neurons are required for signaling the unavailability of touch information, in addition to their conventional role of signaling touch stimulation. Furthermore, we found that the ASE taste neurons, which similarly to the touch neurons, express the FLP-20 neuropeptide, exhibit altered FLP-20 expression levels in a touch-dependent manner, thus cooperating with the touch circuit. These results imply a novel form of neuronal signaling that enables C. elegans to distinguish between lack of touch stimulation and loss of touch sensation, producing adaptive behavioral adjustments that could overcome the inability to detect potential threats.
Rabinowitch I., Upadhyaya B., Pant A., Galski D., Kreines L. and Bai J. (2021) Circumventing neural damage in a C. elegans chemosensory circuit using genetically engineered synapses. Cell Systems 12: 263-271.e4.
Neuronal loss can considerably diminish neural circuit function, impairing normal behavior by disrupting information flow in the circuit. Here, we use genetically engineered electrical synapses to reroute the flow of information in a C. elegans damaged chemosensory circuit in order to restore organism behavior. Our analysis suggests that these additional electrical synapses help restore circuit function by amplifying weakened neuronal signals in the damaged circuit in addition to emulating the wild-type circuit.
Rabinowitch I. (2020) What would a synthetic connectome look like? Physics of Life Reviews 33: 1-15.
As the fields of connectomics and synthetic biology are independently growing, I propose in this review to consider the benefits of combining the two, to create synthetic connectomics, a new form of neuroscience and a new form of synthetic biology. The goal of synthetic connectomics would be to artificially design and construct the connectomes of live behaving organisms.
Rabinowitch I. (2019) Synthetic biology in the brain: a vision of organic robots. Artificial Life Conference Proceedings, MIT Press 654-655.
A main focus of synthetic biology has been the engineering of new gene circuits that can produce artificial cellular functions. I propose to scale up this approach to include, beyond single cells and gene circuits, also entire multi-cellular organisms and the brain circuits that regulate their behavior. Such synthetic biology in the brain will offer new ways for understanding how brain connectivity relates to brain function, and could ultimately lead to futuristic technologies such as neuronally-programmed organic robots or biologically-based brain repair. As a first step towards this ambitious goal I have developed a technique for genetically inserting new synaptic connections into the nervous system of the nematode worm C. elegans, enabling the manipulation of information flow in the nervous system and the reprogramming of whole animal behavior in this organism. This approach may be expanded and adapted to other genetic models, and opens the way to possible new forms of artificial life.
Rabinowitch I., Laurent P., Zhao B., Walker D., Beets I., Schoofs L., Bai J., Schafer W.R. and Treinin M. (2016). Neuropeptide-driven cross-modal plasticity following sensory loss in C. elegans. PLoS Biology 14: e1002348.
The brain has the remarkable capacity to respond to sensory loss by boosting remaining functioning senses. For example, certain features of hearing are improved in blind people. What are the cellular and molecular mechanisms underlying this effect? To simplify these problems, we examined them in an organism with a substantially less complicated nervous system than our own, the roundworm C. elegans. We discovered that C. elegans mutants that cannot sense touch to the body exhibit an improved sense of smell. We were able to pinpoint this change in sensory performance to a change in strength of a specific synapse in the olfactory circuit. We further found that in normal worms, this olfactory synapse is suppressed through a neuropeptide signal transmitted from the touch sensing neurons. In contrast, without any touch input, the touch neurons secrete less neuropeptide, the olfactory synapse becomes stronger, and the sense of smell improves.
Rabinowitch I., Chatzigeorgiou M., Zhao B., Treinin M. and Schafer W.R. (2014). Rewiring neural circuits by the insertion of ectopic electrical synapses in transgenic C. elegans. Nature Communications 5: 4442.
Here we describe a synthetic biology approach to the study of neural circuits, whereby new electrical synapses can be introduced in novel sites in the neuronal circuitry to reprogram behaviour. We added electrical synapses composed of the vertebrate gap junction protein Cx36 between Caenorhabditis elegans chemosensory neurons with opposite intrinsic responses to salt. Connecting these neurons by an ectopic electrical synapse led to a loss of lateral asymmetry and altered chemotaxis behaviour. In a second example, introducing Cx36 into an inhibitory chemical synapse between an olfactory receptor neuron and an interneuron changed the sign of the connection from negative to positive, and abolished the animal’s behavioural response to benzaldehyde. These data demonstrate a synthetic strategy to rewire behavioural circuits by engineering synaptic connectivity in C. elegans.
Rabinowitch I., Chatzigeorgiou M. and Schafer W.R. (2013). A gap junction circuit enhances processing of coincident mechanosensory inputs. Current Biology 23: 963-967.
Electrical synapses have been shown to be important for enabling and detecting neuronal synchrony in both vertebrates and invertebrates. Hub-and-spoke circuits, in which a central hub neuron is electrically coupled to several input neurons, are an overrepresented motif in the C. elegans nervous system and may represent a conserved functional unit. The functional relevance of this configuration has been demonstrated for circuits mediating aggregation behavior and nose touch perception. Modeling approaches have been useful for understanding structurally and dynamically more complex electrical circuits. Therefore, we formulated a simple analytical model with minimal assumptions to obtain insight into the properties of the hub-and-spoke microcircuit motif.
Lena Kreines
Dolev Galski
Ithai started his academic path studying Industrial Engineering at Tel Aviv University, where he learned quantitative approaches to the analysis and design of complex systems. He then steered to neuroscience, embarking on a Ph.D. at the Hebrew University of Jerusalem under the mentorship of Prof. Idan Segev, investigating theoretically how the morphology and structure of neurons determine the dynamic interactions between synaptic inputs. Ithai then continued with postdoctoral training in Cambridge, UK and in Seattle, USA, this time doing experimental work on neural circuits in the tiny nematode worm C. elegans, and gradually bringing together his passion for neurobiology and engineering to establish a neuro-synthetic biology, enabling, for example, the genetic insertion of new synaptic connections into the C. elegans nervous system, thus designing modified or altogether novel behaviors. Ithai joined the Hebrew University’s Faculty of Medicine in late 2017 where his lab is continuously studying and redesigning C. elegans neural circuits.