
Dr., Senior Lecturer
The development of an embryo is an amazing process, in which a single cell develops into a complex, functional organism. Our lab is interested in understanding which genes contribute to normal development of eyes and forebrain, two highly complex organs that enable us to perceive and process information from our environment. Understanding the genetic basis of normal and abnormal forebrain and eye development is necessary for providing genetic counseling, for preventing debilitating congenital malformations and can contribute to developing treatment strategies.
Goal
We seek to better understand the molecular and cellular mechanisms that underlie normal development and congenital malformations of eyes and forebrain.
To achieve this goal, we use genetic and chemical manipulations of zebrafish embryos, which enable us to generate and study these processes in vivo in a vertebrate model organism.
Model system
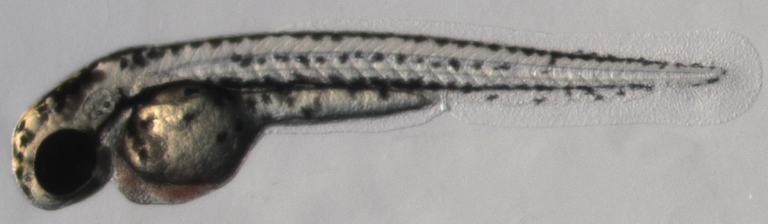
Over the past 3 decades zebrafish have developed as an eminent model for embryonic development, physiology, disease modeling and drug discovery. The zebrafish embryo shows many similarities to other vertebrates and to mammals at various levels, including molecular, cellular and physiological.
Advantages of this model system include fast ex-utero development of embryos, amenability to genetic manipulations, and embryo transparency that allows long-term live imaging at very high resolution.
Methods
A large variety of methods is used in our studies and includes:
Ocular vascularization defects play a major role in several human eye diseases that lead to impaired vision. Hence, understanding the molecular mechanisms that govern normal and abnormal ocular blood vessel development could provide important insights into the mechanisms underlying these diseases.
We are studying two main questions:
1. Is patterning of ocular vessels influenced by eye tissues? The early vasculature of the zebrafish eye is patterned in a highly stereotypic manner. What signals dictate the formation of this pattern? We use transgenic lines, live imaging and chemical manipulations to address this question.
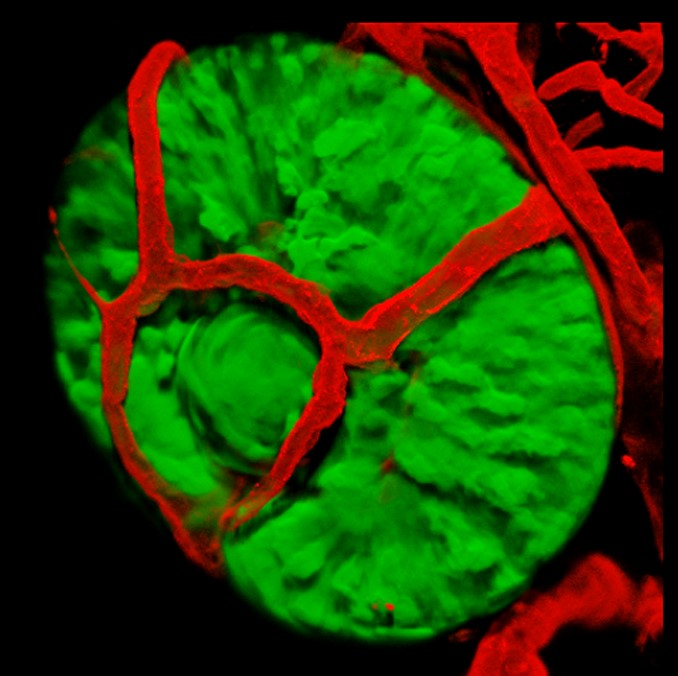
We use genetic mutants with eye and lens malformations as models for abnormal development of these organs. By understanding the mechanisms leading to these malformations we can improve our ability to predict congenital eye problems, and possibly to prevent eye diseases
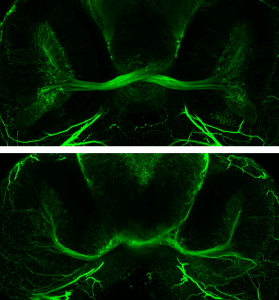
Six3: Mutations in human SIX3 are associated with holoprosencephaly (HPE), the most common forebrain malformation, often including various eye malformations.
We developed a zebrafish model of Six3 loss of function in which embryos show optic disc coloboma, abnormal optic nerve development and deficiencies in the ventral midline of the forebrain. This model can now be used to further identify specific defects in the holoprosencephalic brain, and to better understand the roles of Six3 in eye morphogenesis and in the pathogenesis of HPE.
Secondary cataract is the main complication of cataract surgeries, millions of which are carried out every year worldwide. We have identified a genetic mutation leading to development of lens abnormality with features of secondary cataract, driven by excessive TGFb signaling. We use this new model to screen for drugs that can interfere with pathological processes driven by TGFb signaling, not only in the lens but in other organs as well.

ISF – Israel Science Foundation, European Commission, ICRF – Israel Cancer Research Fund, MOH – Ministry of Health, NIH – National Institute of Health, Barry and Esther Naiberg.
Transcriptome analysis of atad3-null zebrafish embryos elucidates possible disease mechanisms
Ezer, S., Ronin, N., Yanovsky-Dagan, S., Rotem-Bamberger, S., Halstuk, O., Wexler, Y., … & Harel, T. (2024). Transcriptome analysis of atad3-null zebrafish embryos elucidates possible disease mechanisms. Orphanet J Rare Dis.
ATAD3A, a nuclear gene encoding the ATAD3A protein, has diverse roles in mitochondrial processes, encompassing mitochondrial dynamics, mitochondrial DNA maintenance, metabolic pathways and inter-organellar interactions. Pathogenic variants in this gene cause neurological diseases in humans with recognizable genotype-phenotype correlations. Yet, gaps in knowledge remain regarding the underlying pathogenesis. In our study, the atad3-null zebrafish model emerges as a reliable representation of human ATAD3A-associated disorders, with similarities in differentially expressed pathways and processes. Furthermore, our study underscores mitochondrial dysfunction as the primary underlying pathogenic mechanism in ATAD3A-associated disorders and identifies potential readouts for therapeutic studies.
Selective Requirements for Vascular Endothelial Cells and Circulating Factors in the Regulation of Retinal Neurogenesis
Dhakal S, Rotem-Bamberger S, Sejd JR, Sebbagh M, Ronin N, Frey RA, Beitsch M, Batty M, Taler K, Blackerby JF, Inbal A, Stenkamp DL. (2021). Front Cell Dev Biol. 9:628737.
In the present study we use the informative embryonic zebrafish model, with strategies that selectively target components of the vascular system, to unravel the contributions of endothelial cells vs. circulating factors for supporting and regulating retinal development. We find that the presence of endothelial cells is needed for retinal organization and retinal cell survival, while the presence of circulation – likely contributing circulating factors – also participates in the control of retinal cell proliferation and retinal cell differentiation. One of our strategies specifically disrupted the vasculature from 48 hpf to 72 hpf, a period of expansion of the ocular vasculature (Hartsock et al., 2014), and retinal differentiation (Raymond et al., 1995; Hu and Easter, 1999). This temporally-selective ablation of vessels produced a near-phenocopy of the cloche mutant retinal phenotype, suggesting this developmental window is a key period of vessel-retina interactions.
Six3 regulates optic nerve development via multiple mechanisms.
Samuel A, Rubinstein AM, Azar TT, Ben-Moshe Livne Z, Kim SH, Inbal A. (2016). Sci Rep. 6:20267.
Malformations of the optic nerve lead to reduced vision or even blindness. During optic nerve development, retinal ganglion cell (RGC) axons navigate across the retina, exit the eye to the optic stalk (OS) and cross the diencephalon midline at the optic chiasm en route to their brain targets. Many signalling molecules have been implicated in guiding various steps of optic nerve pathfinding, however much less is known about transcription factors regulating this process. Here we show that in zebrafish, reduced function of transcription factor Six3 results in optic nerve hypoplasia and a wide repertoire of RGC axon pathfinding errors. These abnormalities are caused by multiple mechanisms, including abnormal eye and OS patterning and morphogenesis, abnormal expression of signalling molecules both in RGCs and in their environment and anatomical deficiency in the diencephalic preoptic area, where the optic chiasm normally forms. Our findings reveal new roles for Six3 in eye development and are consistent with known phenotypes of reduced SIX3 function in humans. Hence, the new zebrafish model for Six3 loss of function furthers our understanding of the mechanisms governing optic nerve development and Six3-mediated eye and forebrain malformations.
Abnormal retinal development in Cloche mutant zebrafish.
Dhakal S, Stevens CB, Sebbagh M, Weiss O, Frey RA, Adamson S, Shelden EA, Inbal A, Stenkamp DL. (2015) Dev Dyn. 244(11):1439-1455.
We use an avascular zebrafish embryo, cloche−/− (clo−/−), to begin to identify necessary developmental functions of the ocular vasculature in regulating development and patterning of the neural retina, in vivo. These studies are possible in zebrafish embryos, which do not yet rely upon the vasculature for tissue oxygenation. clo−/− embryos lacked early ocular vasculature and were microphthalmic, with reduced retinal cell proliferation and cell survival. Retinas of clo mutants were disorganized. This phenotype is consistent with a neural and glial developmental patterning role for the early ocular vasculature that is independent of its eventual function in gas exchange.
Development and origins of zebrafish ocular vasculature.
Kaufman R, Weiss O, Sebbagh M, Ravid R, Gibbs-Bar L, Yaniv K, Inbal A. (2015). BMC Dev Biol. 15:18.
The developing eye receives blood supply from two vascular systems, the intraocular hyaloid system and the superficial choroidal vessels. In zebrafish, a highly stereotypic and simple set of vessels develops on the surface of the eye prior to development of choroidal vessels. The origins and formation of this so-called superficial system have not been described.
We have analyzed the development of superficial vessels by time-lapse imaging and identified their origins by photoconversion experiments in kdrl:Kaede transgenic embryos. We show that the entire superficial system is derived from a venous origin, and surprisingly, we find that the hyaloid system has, in addition to its previously described arterial origin, a venous origin for specific vessels. Despite arising solely from a vein, one of the vessels in the superficial system, the nasal radial vessel (NRV), appears to acquire an arterial identity while growing over the nasal aspect of the eye and this happens in a blood flow-independent manner.
Our results provide a thorough analysis of the early development and origins of zebrafish ocular vessels and establish the superficial vasculature as a model for studying vascular patterning in the context of the developing eye.

Lab manager
The crosstalk between the eye development and the molecular mechanisms of the ocular blood vessel development

M.Sc. Student
In my project we are looking for potential treatments that may rescue and prevent PCO after cataract surgery using a lh3 deficient zebrafish as a model.

undergraduate student
The effects of the cloche and silent heart mutations on the development rate of embryo eye size using the Zebrafish model.

Undergraduate student
Examining genetic models in zebrafish, experiment documentation and fish husbandry.
Soon after finishing high school, I started my academic career at the Hebrew University of Jerusalem, where I studied Dentistry and in parallel obtained an M.Sc. in oral biology. Having served in the army as a dentist, I realized that I preferred to do research over practicing dentistry. I therefore decided to completely change course and joined the laboratory of Adi Salzberg at the Technion, to study embryonic development of the nervous system, using Drosophila as a model organism. I was fascinated by the amazing processes of embryonic development and continued to do a postdoc at Vanderbilt University in the laboratory of Lila Solnica-Krezel, where I switched to working on zebrafish and studying development of the forebrain.
In 2009 I joined the Hebrew University Medical School and started my laboratory, where we study eye development in zebrafish, and develop models for human congenital diseases that involve the eyes.