
Cecile and Seymour Alpert Professor in Pain Research
Pain is a multidimensional and multilevel experience involving complex sensory processing modulated by emotional and cognitive operations. This complexity exists in both adaptive pain as well as in maladaptive embodiments of pain, where pain occurs without obvious stimuli. The ability of pain systems to adapt to changes in stimuli characteristics, and, therefore, to shift the stimulus-response curve at each level of processing adds to this intricacy. Maladaptive functional or neuropathic pain represents the highest level of complexity since there is no defined stimulus that initiates the process; yet once initiated, the pain becomes perpetual due to multilevel plastic changes occurring from the primary sensory neurons all the way to the cerebral cortex. Inadequate comprehension of pain mechanisms is likely the reason that chronic pain has been implicated as the most prevalent unmet medical need. Pain presents a significant and costly liability to workers, employers and society. Yet of highest importance is the daily perpetual misery and suffering, which was perhaps best expressed by Milton in Paradise Lost: “Pain is perfect misery, the worst of Evils, and, excessive, overturns all patience.” Lack of adequate treatments for pain has a profound impact on the quality of life and health-associated costs. A comprehensive and detailed understanding of the neurobiology of pain is urgently required to enable the development of more effective treatments.
In our lab are aiming to understand the complex phenomenon of pain. We are studying molecular and cellular mechanisms of normal and abnormal (chronic) pain at all levels starting from the terminal endings of peripheral neurons at the target organs and all the way up to cerebral cortex. We also developing new approaches for pain-selective anesthesia – an effective pain treatment without side effects. In our lab we are using in vivo and in vitro electrophysiology and imaging paired with computational and behavioral approaches.
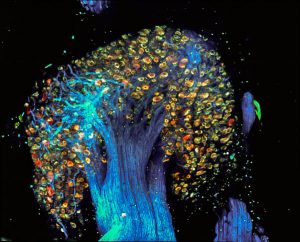
“Tree of Pain” – Image of Dorsal Root Ganglion labelled by PainBow
Pain is a multilevel experience involving complex sensory processing modulated by emotional and cognitive operations. This complexity exists in both adaptive pain as well as in maladaptive embodiments of pain, where pain occurs without obvious noxious stimuli. The ability of pain systems to adapt to changes in stimuli characteristics, and, therefore, to shift the stimulus-mediated response at each level of processing adds to this intricacy. Maladaptive functional or neuropathic pain represents the highest level of complexity since there is no defined stimulus that initiates the process; yet once initiated, the pain becomes perpetual due to multilevel plastic changes occurring from the nociceptors all the way to the cerebral cortex. Inadequate comprehension of pain mechanisms is likely the reason that chronic pain has been implicated as the most prevalent unmet medical need.
My lab is studying molecular and cellular mechanisms of normal and abnormal (chronic) pain at all levels, starting from the terminal endings of peripheral neurons at the target organs; spinal cord and all the way up to cerebral cortex. We are using advanced genetic, molecular, electro-optical, computational, and behavioral approaches to study the plasticity of pain-related circuits along the pain neuraxis leading to chronic pain. Recently we have developed an approach allowing for the first time to monitor the activity of peripheral terminals in vivo in intact tissue. We characterized the changes in encoding of pain-inducing stimuli by nociceptive terminals underlying abnormal pain. We then explore how the pain-related spinal neuronal network integrates pain-related information from the terminals of primary afferent neurons. We also ask how this network integration is changing during pathological conditions when the input from the periphery is modified. Using cutting-edge in vivo imaging from pain-related spinal cord neurons, we are recording the “behavior” of many spinal neurons at once following noxious stimulation to the paw. We then analyze how local injury or activation of descending pain modulation system alters the activity of central neuronal networks, leading to pathological pain.Our lab is also studying mechanisms of visceral pain and developing new approaches to diminish pain and itch without side effects.
Many therapeutic procedures would be intolerable without the use of local anesthesia which reversibly eliminates sensation conducted along peripheral nerves. By blockade of sodium channels, local anesthetics prevent the transmission of pain; however, since local anesthetics act unselectively on all types of neurons, they also cause loss of non-painful sensation, motor paralysis, and autonomic block. Thus, the approaches that will allow selective blocking of pain fibers are of great clinical importance. We have developed a novel platform for pain selective anesthesia using the nociceptive transducer channels TRPV1 and TRPA1 as a “natural” drug delivery system for introducing a membrane-impermeable and therefore clinically ineffective lidocaine derivative QX-314 into nociceptive (pain-related) neurons1. We showed that the injection of QX-314 and capsaicin/AITC in-vivo together, but not alone, abolished the response to noxious mechanical and thermal stimuli without any motor or tactile deficits1. This approach for selective targeting of sodium channel blockers through nociceptive-specific channels could potentially be used clinically to produce long-lasting regional analgesia whilst preserving motor and autonomic function. We have continued to develop this approach and demonstrated that it represents a novel concept of targeted delivery of impermeant compounds and can be used not only to block activity but also modulate intracellular signal transduction and metabolism pathways in pain-sensing neurons a well as cancer2 or any other TRP-expressing cells3, while minimizing effects on other types of cells. This approach has been adopted by peers in academia and the pharmaceutical industry worldwide. We and others have demonstrated that it can prospectively be used clinically to produce long-lasting regional analgesia while preserving motor and autonomic function. In addition to the application of this technology for surgery and childbirth, this technique could also be used to diminish itch and postoperative, cancer, inflammatory, and neuropathic pain as well as selectively ablate cancer cells. We are currently exploring the use of this approach for blockade of Inflammatory Bowel Disease(IBD)-mediated gut pain and optimizing this approach for clinical use.
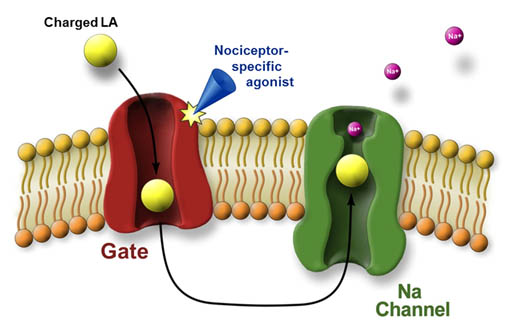
Figure 1. Targeted delivery of charged local anesthetics (LA) into pain-related (nociceptive) peripheral neurons via the pore of nociceptive specific TRPV1 or TRPA1 channels
(1). Binshtok AM, Bean BP, and Woolf CJ. (2007). Inhibition of nociceptors by TRPV1-mediated entry of impermeant sodium channel blockers. Nature. 449 (7162): 607-611
(2). Neumann-Raizel H, Shilo A, Lev S, Mogilevsky M, Katz B, Shneor D, Shaul YD, Leffler A, Gabizon A, Karni R, Honigman A, and Binshtok AM (2019). 2-APB and CBD-mediated targeting of charged cytotoxic compounds into tumor-like cells suggests the involvement of TRPV2 channels. Frontiers in Pharmacology; Oct 15;10:1198
(3). Roberson DP, Gudes S, Sprague J, Patoski HAW, Robson VK, Blasl F, Duan B, Oh SB, Bean BP, Ma Q, Binshtok AM *, Woolf CJ *. (2013). Activity dependent silencing reveals functionally distinct itch-generating sensory neurons. * Co-corresponding and co-senior authors. Nature Neuroscience, 16(7):910-8.
Nociceptive free nerve endings are specialized for the detection of harmful stimuli. Since these tiny structures are the most physiologically relevant site of pain detection, understanding the molecular network underlying nociceptive terminal functionality and its modulation in different pain states is imperative for understanding mechanisms of normal and pathological pain. Yet, these nociceptive terminals are inaccessible by conventional methodologies due to their miniature size. Consequently, the question of how nociceptive neurons process noxious stimuli in normal and pathological conditions remains obscure. To overcome these limitations, my team has developed an optical approach allowing, for the first time, to detail the mechanism of noxious stimuli coding by nociceptive terminals in vivo in intact tissue in normal and pathological conditions1. Using this approach, we have determined the location of the Spike Initiation Zone (SIZ) along the nociceptive terminal fiber. We showed that the location of SIZ depends on the functional availability of sodium channels. Importantly, we have discovered that during inflammation, the location of the SIZ is shifted towards the terminal end1. Using a computational approach, we demonstrated that this shift in the location of SIZ underlies an inflammation-mediated increase in pain1. The results of these work are crucial for understanding how peripheral pain neurons process information in normal and pathological states, potentially forming the basis for more effective and specific pain treatments. Moreover, the platform that we developed is believed by peers to be a new gold standard approach for studying signal detection by nociceptive and other sensory neurons. We are now using this platform to study the changes in the molecular machinery of the nociceptive terminals participating in the detection of noxious underlying pathological pain. Moreover, recently we developed a novel computational model of primary afferent pain-related neurons to study how nociceptive neurons integrate noxious information. We show that the integration of noxious information depends on the morphology of the terminal arborizations of the primary nociceptive neurons and described how this integration and, consequently, the neuronal output change under pathologic conditions2. Our findings help to predict how nociceptive neurons encode noxious stimuli and how this encoding changes in pathologic conditions, leading to pain.
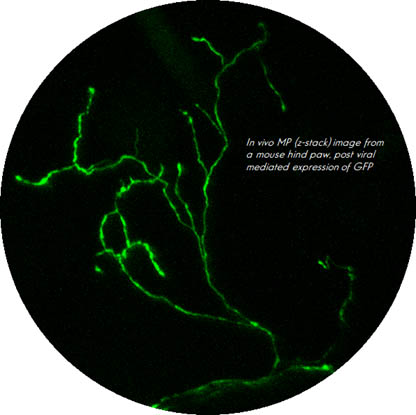
Figure 2. Image of the mice nociceptive terminal in vivo
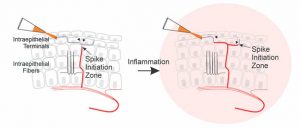
Figure 3. Shift ion Spike Initiation Zone towards the terminal end leads to an increase in nociceptive activity, leading to inflammatory pain
(1). Goldstein R, Barkai O, Íñigo-Portugués A, Katz B, Lev S, Binshtok AM (2019). Location and Plasticity of the Sodium Spike Initiation Zone in Nociceptive Terminals in vivo. Neuron, Volume 102, Issue 4, 22 May 2019, Pages 801-812.
(2). Barkai O, Butterman R, Katz B, Lev S, Binshtok AM (2020). The input-output relation of primary nociceptive neurons is determined by the morphology of the peripheral nociceptive terminals. Journal of Neuroscience, 2020 Dec 2;40(49):9346-9363
We, in collaboration with Prof. Lichtman, have developed a novel multispectral labeling technique to parse projections of many neurons simultaneously. This technique entails injection, in overlapping regions of an innervated organ, with three or more different colored retrograde tracers1. These tracers undergo vesicular uptake by nerve terminals and retrogradely transport to the neuronal soma. Based on the combinations and intensities of the colors in the individual vesicles transported to the cell soma, we calculate the projection sites of the neuron’s axon. This neuronal positioning system (NPS) enables mapping of many axons in a simple automated way1. Recently we have modified this approach to study the innervation patterns of peripheral pain-related neurons and we called it Painbow2. We used this approach to examine how these innervation patterns are changing following post-nerve injury reinnervation An injury to peripheral nerves leads to skin denervation, followed by skin reinnervation. Importantly, the reinnervation stage is accompanied by increased pain sensitivity of the previously denervated areas and the development of neuropathic pain. Although the central mechanisms of neuropathic pain are widely studied, the role of abnormal reinnervation in the development of neuropathic pain is still obscured. Our results suggest that local changes in peripheral innervation following denervation contribute to neuropathic pain development2.
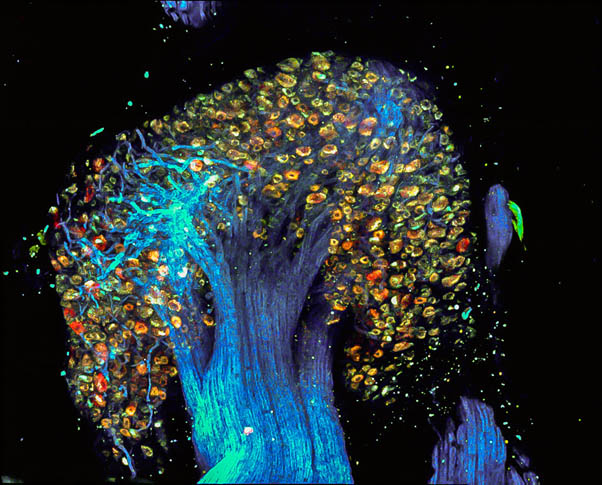
Figure 4. Dorsal Root Ganglion labeled by the PainBow showing cell bodies and axons of sensory neurons
(1). Tsuriel S., Gudes S., Draft RW, Binshtok AM *, Lichtman JW * (2015). Multispectral labeling technique to map many neighboring axonal projections in the same tissue. * Co-corresponding and co-senior authors. Nature Methods, 12(6):547-52.
(2). Leibovich H, Buzaglo N, Tsuriel S, Peretz L, Caspi Y, Katz B, Lev S, Lichtstein D, and Binshtok AM (2020). Abnormal reinnervation of denervated areas following nerve injury facilitates neuropathic pain. Cells; Apr 18;9(4):1007
Pain hypersensitivity is a common feature of inflammation. A variety of mediators released by immune and injured cells during inflammation act on nociceptive neurons and increase their excitability, thus leading to inflammatory pain. The understanding of the mechanisms of proinflammatory mediators-induced nociceptive hyperexcitability is of paramount importance for the development of an efficient therapeutic. During my postdoctoral fellowship, I led a project showing that pro-inflammatory cytokine IL-1b directly activates nociceptive neurons by increasing the functional availability of sodium channels1. Now, as a PI, my group, using electrophysiological and behavioral approaches, have shown that another pro-inflammatory cytokine, TNFa leads to inflammatory pain also by increasing the functional availability of sodium channels2. Using electrophysiological, computational and behavioral approaches, we showed that potassium Kv7/M channels are crucial in determining the nociceptive excitability. Using biochemical, immunohistochemical, electrophysiological and behavioral approaches, we have discovered a novel pro-inflammatory mediator, PDGF, which is released during acute inflammation and by blocking Kv7/M channels increases nociceptive excitability and facilitates inflammatory pain4. We showed that blockade of the receptor to PDGF, by clinically used anticancer compound, Imatinib, significantly reduces inflammatory pain4.
(1). Binshtok AM, Wang H, Zimmermann K, Amaya F, Vardeh D, Shi L, Brenner G, Ji RR, Bean BP, Woolf CJ, Samad TA. (2008). Nociceptors are interleukin-1b sensors. Journal of Neuroscience. 28(52):14062-14073.
(2). Gudes S, Barkai O, Caspi Y, Katz B, Lev S, Binshtok AM (2015). The Role of Slow and Persistent TTX-resistant Sodium Currents in Acute Tumor Necrosis Factor a – Mediated Increase in Nociceptors Excitability. Journal of Neurophysiology, 113(2): 601-19
(3). Barkai O, Goldstein R, Caspi Y, Katz B, Lev S and Binshtok AM (2017). The role of Kv7/M potassium channels in controlling ectopic firing in nociceptors. Frontiers in Molecular Neuroscience, 2017 Jun 13; 10:181
(4). Barkai O, Piug S, Lev S, Title B, Katz B, Eli-Berchoer L, Gutstein H, Binshtok AM (2019). Platelet-derived growth factor activates nociceptive neurons by inhibiting M-current and contributes to inflammatory pain. Pain, 160(6): 1281-1296
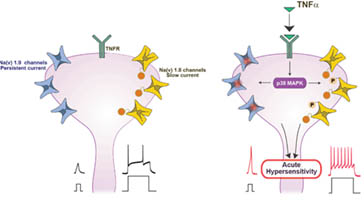
FiFigure 5. An increase in sodium channel availability leads to nociceptive hyperexcitability underlying inflammatory pain
The peripheral nociceptive neurons encode noxious information and relay it to the superficial laminae of the dorsal spinal cord. There, the information regarding the noxious stimuli enters the complex nociceptive neuronal network, which is composed of projection neurons and excitatory and inhibitory interneurons. The output from this network is transmitted into higher brain centers sculpturing the sensation of pain. In pathological states, such as tissue inflammation, the input entering the spinal cord nociceptive network from the inflamed tissue is changing, affecting the activity of the network. Understanding these changes in the nociceptive spinal cord network activity is crucial for understanding the mechanisms of pathological pain. We are studying how the pain-related spinal neuronal network integrates pain-related information from primary afferent neurons. We also ask how this network integration is changing during pathological conditions when the input from the periphery is modified. Using cutting-edge in vivo imaging approach, we are recording the “behavior” of many spinal neurons at once in vivo following noxious stimulation to the paw. We then generate a local paw inflammation and characterize the same neuronal network’s response to the same stimuli but in the inflammatory conditions. This experimental approach allows us, for the first time, to study how injury alters the activity of central neuronal networks, leading to pathological pain.
Our previous and current funding sources include European Research Foundation (ERC), German Research Foundation (DFG), Israeli Science Foundation (ISF), Canadian Institute of Health Research and Israeli Science Foundation, Rosetrees Trust, TEVA’s National Network of Excellence in Neuroscience.
Babu, T., Muthuramalingam, R. P. K., Chng, W. H., Yau, J. N. N., Acharya, S., Engelmayer, N., Feldman-Goriachnik R., Lev S., Pastorin G., Binshtok, A., Hanani M. & Gibson, D. (2025). Multitargeting Pt (IV) Derivatives of Cisplatin or Oxaliplatin Inhibit Tumor Growth in Mice without Inducing Neuropathic Pain. Journal of Medicinal Chemistry.
https://pubs.acs.org/doi/10.1021/acs.jmedchem.4c02263
Mazor, Y., Engelmayer, N., Nashashibi, H., Rottenfußer, L., Lev, S., & Binshtok, A. M. (2024). Attenuation of Colitis-Induced Visceral Hypersensitivity and Pain by Selective Silencing of TRPV1-Expressing Fibers in Rat Colon. Inflammatory Bowel Diseases. https://doi.org/10.1093/ibd/izae036
Dey, S., Barkai, O., Gokhman, I., Suissa, S., Haffner-Krausz, R., Wigoda, N., … Binshtok, A. & Yaron, A. (2023). Kinesin family member 2A gates nociception. Cell Reports.
https://doi.org/10.1016/j.celrep.2023.113257
Rayi, P.R, Lev, S. and Binshtok, A.M. (2023). Age-dependent decrease in inhibitory drive on the excitatory superficial spinal dorsal horn neurons. Neurobiol Pain. https://doi.org/10.1016/j.ynpai.2023.100139
Katz, B., Zaguri, R., Edvardson, S., Maayan, C., Elpeleg, O., Lev, S., Davidson, E., Peters, M., Kfir-Erenfeld, S., Berger, E., Ghazalin, S., Binshtok, A.M., and Minke, B. (2023). Nociception and pain in humans lacking functional TRPV1 channel. J Clin. Invest. https://doi.org/10.1172/JCI153558
Caspi, Y., Mazar, M., Kushnir, Y., Mazor, Y., Katz, B., Lev, S., & Binshtok, A. M. (2022). Structural plasticity of axon initial segment in spinal cord neurons underlies inflammatory pain. Pain https://doi.org/10.1097/j.pain.0000000000002829
Barkai, O., Rayi, P. R., Butterman, R., Katz, B., Lev, S., & Binshtok, A. M. (2022). Encoding of inflammatory hyperalgesia in mice spinal cord. Pain, 10-1097. https://doi.org/10.1097/j.pain.0000000000002727
Wong, C., Barkai, O., Wang, F., Pérez, C. T., Lev, S., Cai, W., … & Khoutorsky, A. (2022). mTORC2 mediates structural plasticity in distal nociceptive endings that contributes to pain hypersensitivity following inflammation. The Journal of Clinical Investigation. https://doi.org/10.1172/JCI152635
In this collaborative work with Dr. Khoutorsky lab at McGill, we showed that tissue inflammation leads to an activation of the mammalian target of rapamycin complex 2 (mTORC2). We also showed that this Inflammation-induced activation mTORC2 triggers changes in the architecture of nociceptive terminals, which are sufficient to increase the excitability of nociceptors, thus leading to inflammatory pain. We showed that tissue inflammation-induced activation of mTORC2 causes structural plasticity of nociceptive free nerve endings in the epidermis and inflammatory hyperalgesia, representing a therapeutic target for inflammatory pain.
Gershon, D., Negev-Goldstein, R. H., Abd al Razzaq, L., Lev, S., & Binshtok, A. M. (2022). In vivo optical recordings of ion dynamics in mouse corneal primary nociceptive terminals. STAR protocols, 3(1), 101224.
In this paper, we describe a protocol that allows us to measure ion dynamics in nociceptive terminal endings in intact tissue in vivo, allowing measuring nociceptive terminal activity in physiological conditions. The fast and high-resolution optical recording technique described here provides a tool for the detailed study of a nociceptive terminal’s functional molecular network underlying noxious stimuli detection and transmission in normal and pathological conditions. Moreover, this platform can be utilized to enable studying the terminals of other neuronal types, such as other primary sensory neuron terminals.
Barkai O, Butterman R, Katz B, Lev S and Binshtok AM (2020). The input-output relation of primary nociceptive neurons is determined by the morphology of the peripheral nociceptive terminals. The Journal of Neuroscience, Dec 2;40(49):9346-9363. doi: 10.1523/JNEUROSCI.1546-20.2020; Accompanied by Editorial; Featured in the Journal of Neuroscience Spotlight – the article is chosen among the published articles that receive the strongest reviews in 2020
In the canonical neuron, spikes are initiated near the base of axons and propagate to the terminal arbor to induce transmitter release. In sensory neurons, however, spikes are initiated in the peripheral axon terminals, propagate toward a branch point near the soma, then continue along the central branch to a central terminal arbor in the spinal cord. Notably, nociceptor neurons can have complex peripheral arbors with multiple spike initiating zones. Barkai et al. created a computational model to investigate how all-or-nothing spikes generated in separate peripheral branches are integrated at branch points. Their results revealed that the input–output function of nociceptor neurons is strongly influenced by the morphology of the peripheral arbor—a characteristic that varies considerably across cells.
Goldstein R, Barkai O, Íñigo-Portugués A, Katz B, Lev S, Binshtok AM (2019). Location and Plasticity of the Sodium Spike Initiation Zone in Nociceptive Terminals in vivo. Neuron, Volume 102, Issue 4, 22 May 2019, Pages 801-812.e5; doi: 10.1016/j.neuron.2019.03.005. Accompanied by Editorial
We have developed an optical approach allowing, for the first time, to detail the mechanism of noxious stimuli coding by nociceptive terminals in vivo in intact tissue in normal and pathological conditions. Using this approach, we have determined the location of the Spike Initiation Zone (SIZ) along the nociceptive terminal fiber. We showed that the location of SIZ depends on the functional availability of sodium channels. Importantly, we have discovered that during inflammation, the location of the SIZ is shifted towards the terminal end. Using a computational approach, we demonstrated that this shift in the location of SIZ underlies an inflammation-mediated increase in pain.
Barkai O, Piug S, Lev S, Title B, Katz B, Eli-Berchoer L, Gutstein H, Binshtok AM (2019). Platelet-derived growth factor activates nociceptive neurons by inhibiting M-current and contributes to inflammatory pain. Pain, 160(6): 1281-1296
We have discovered a novel pro-inflammatory mediator, PDGF, which is released during acute inflammation and increases nociceptive excitability and facilitates inflammatory pain by blocking Kv7/M channels. We demonstrated that blockade of the receptor to PDGF by clinically used anticancer compound – imatinib – significantly reduces inflammatory pain.
Tsuriel S. , Gudes S. , Draft RW , Binshtok AM*, Lichtman JW * (2015). A multispectral labeling technique to map many neighboring axonal projections in the same tissue. Nature Methods, 2015 June; 12(6):547-52. *co-corresponding authors
We, in collaboration with Prof. Lichtman, have developed a novel multispectral labeling technique to parse projections of many neurons simultaneously. This technique entails injection, in overlapping regions of an innervated organ, with three or more different colored retrograde tracers. These tracers undergo vesicular uptake by nerve terminals and retrogradely transport to the neuronal soma. Based on the combinations and intensities of the colors in the individual vesicles transported to the cell soma, we calculate the projection sites of the neuron’s axon. This neuronal positioning system (NPS) enables the mapping of many axons in a simple automated way.
Roberson DP , Gudes S , Sprague J , Patoski HAW , Robson VK , Blasl F , Duan B , Oh SB , Bean BP , Ma Q, Binshtok AM *, Woolf CJI*. (2013). Activity dependent silencing reveals functionally distinct itch-generating sensory neurons.
Nature Neuroscience, 2013 Jul; 16(7):910-8. Accompanied with News and Views. *co-corresponding authors
Itch is a complex, unpleasant cutaneous sensation that in some respects resembles pain, yet different in terms of its intrinsic sensory quality and the urge to scratch. The molecular factors underlying the detection of itch-related stimuli have been identified – Co-activation of TRPV1 and histamine receptor H1R is required to produce histamine-dependent itch, and that of TRPA1 and MrgprA3 for the non-histamine-mediated itch. It remains unclear, however, whether these distinct molecular machinery are expressed by the same or different afferent fibers. This distinction is clinically important since therapies targeting neuronal activity exclusively of histaminergic itch fibers might be therapeutically ineffective for the treatment of non-histaminergic itch if the neurons mediating the two itches are functionally distinct in the mature nervous system. To study if histamine and non-histamine-mediated itch are functionally distinct, we have utilized a strategy of silencing specific subsets of pruritogen-sensitive primary sensory fibers by delivery of a charged sodium channel blocker through active large pore channels to demonstrate that functional blockade of the sensory fibers that mediate histamine itch does not affect the histamine-independent itch evoked by chloroquine and vice versa. Moreover, we demonstrate that a targeted silencing of itch-generating fibers does not reduce pain-associated behavior. These findings both support the presence of functionally distinct sets of fibers for producing histaminergic and non-histaminergic itch behavior in adult mice and suggest that the targeted silencing of activated sensory fibers may represent a clinically useful anti-pruritic therapeutic approach.
Binshtok AM, Wang H, Zimmermann K, Amaya F, Vardeh D, Shi L, Brenner G, Ji RRC, Bean BP, Woolf CJ, Samad TA. (2008). Nociceptors are interleukin-1b sensors. Journal of Neuroscience. 28(52):14062-14073. Accompanied by Editorial “This week in Journal”
Pain hypersensitivity is a common feature of inflammation. A variety of mediators released by immune and injured cells during inflammation act on nociceptive neurons and increase their excitability, thus leading to inflammatory pain. The understanding of the mechanisms of pro-inflammatory mediators-induced nociceptive hyperexcitability is of paramount importance for the development of an efficient therapeutic. Here we show that pro-inflammatory cytokine IL-1beta directly activates nociceptive neurons by increasing the functional availability of sodium channels.
Binshtok AM, Bean BP, and Woolf CJ. (2007). Inhibition of nociceptors by TRPV1-mediated entry of impermeant sodium channel blockers. Nature. 449 (7162): 607-611. . Accompanied by News and Views. Rated “Exceptional” and gained a factor of 11.2 by “Faculty of 1000 Medicine” – their second highest ranked research publication of all time. Rated “Exceptional” with factor of 10.4 in “Faculty of 1000 Biology” – all time top 25 ranking
We discovered a novel approach to selectively block pain-related (nociceptive) neurons without the undesired side effects of numbness or motor paralysis, which are produced by conventional local anesthetic agents used presently. We developed a nociceptor-specific drug delivery system that exploited activated TRPV1 channels, the nociceptor-specific noxious thermo-sensitive transducer, to target charged (and therefore membrane impermeant) drug molecules selectively into pain-sensing neurons. We found that the pore of activated TRPV1 channels is large enough to pass QX-314, a charged derivative of lidocaine that is ineffective from the outside but blocks sodium channels (and therefore excitability) from the inside of cells. Thus, activation of TRPV1 channels, paired with co-application of otherwise clinically ineffective QX-314, produces selective inhibition of pain-related signals while sparing motor or sensory modalities. This novel approach shows clinical promise as long-lasting regional analgesia that will preserve normal motor and sensory functions and has been adopted by peers in academia and the pharmaceutical industry worldwide.

Postdoctoral Fellow
Characterization of the PDGF-mediated biophysical and molecular effects on nociceptive neurons causing morphine tolerance.

Master student
I am studying the contribution of T type calcium channels to the detection of noxious stimuli by nociceptive terminals in vivo.

PhD Student
Encoding of noxious stimuli by medullary dorsal horn neural circuitry in naive and pathological conditions.

Master
Pain-related spinal cord network activity.

Undergraduate Student in Biomedical Sciences
Selective blockade of visceral pain

Research Associate, lab manager, teaching administrator
I research basic aspects of pain nociception in terms of signal transduction and biophysical properties.

Undergraduate student for Biomedical sciences
Exploring the mechanisms of AIS on neural excitability.

PhD student
Pain signal propagation and local expansion in corneal nociceptors in normal and inflammatory conditions.

PhD student
Pain selective local anaesthesia, bridging the bench to bedside gap.

Postdoctoral Fellow
Visceral perception and pain in functional and inflammatory bowel disease.

Msc
Membrane voltage changes in pain fibers terminals, during normal and pathological state.

PhD Student, Postdoctoral Fellow
The encoding of pain transmission from the peripheral to the central nervous system in normal and pathological conditions using experimental and computational approaches.
We are looking for excellent postdocs and students.




Website designed by toornet
My interest in mechanisms of pain sparked during my undergraduate studies in physiotherapy. Throughout the ten years in which I practiced clinical physiotherapy, I focused on treating patients experiencing pain. My academic interest in pain mechanisms became the driving force of my professional development. I began my graduate studies in order to acquire the knowledge, training, and professional groundwork required to investigate fundamental mechanisms of different forms of pain. In this work, I used whole-cell and single-channel patch-clamp approaches to study the intrinsic and synaptic properties of neurons in the primary somatosensory cortex.
After completing my doctoral degree, summa cum laude, I accepted a postdoctoral fellowship with world-renowned pain neurobiologist Dr. Clifford Woolf at Harvard Medical School. There, my research focused on the physiology of primary nociceptive neurons. My colleagues and I discovered a novel approach to selectively block pain-related (nociceptive) neurons without the undesired side effects of numbness or motor paralysis, which are produced by conventional local anesthetic agents used presently. We developed a nociceptor-specific drug delivery system that exploited activated TRPV1 channels, the nociceptor-specific noxious thermo-sensitive transducer, to target charged (and therefore membrane impermeant) drug molecules selectively into pain-sensing neurons. We found that the pore of activated TRPV1 channels is large enough to pass QX-314, a charged derivative of lidocaine that is ineffective from the outside but blocks sodium channels (and therefore excitability) from the inside of cells. Thus, activation of TRPV1 channels, paired with co-application of otherwise clinically ineffective QX-314, produces selective inhibition of pain-related signals while sparing motor or sensory modalities. This novel approach shows clinical promise as long-lasting regional analgesia that will preserve normal motor and sensory functions and has been adopted by peers in academia and the pharmaceutical industry worldwide. The results of this study were published in Nature, and the article was rated by the Faculty of 1000 Medicine as “Exceptional: A landmark paper representing the top 1% of publications” and was the second highest-ranked research article of all time in all categories of medicine. Subsequently, as a postdoctoral fellow and as Instructor of Anesthesia at Harvard Medical School, I continued exploring molecular mechanisms underlying pain and published my key findings describing a unique connection between the immune and pain systems in the Journal of Neuroscience and Anesthesiology.
As a PI at Hebrew University, I have put together a multidisciplinary research group trained and equipped with cutting edge technologies such as electrophysiology, in vivo two-photon and bright field imaging, as well as computational and behavioral approaches. Together, we aim to analyze neuronal and circuit functions at different levels of pain perception in normal and pathological conditions. We are also developing new approaches for pain and itch-selective anesthesia.
My name is Yoav Mazor. I completed my medical training in the Rappaport Faculty of Medicine of the Technion – Israel Institute of Technology, and my advanced training in Internal Medicine and Gastroenterology in the Rambam Health Care Campus in Haifa. Taking an interest in functional bowel disease, I trained as a fellow in the Neurogastroenterology Unit, Royal North Shore Hospital, Sydney, Australia and concurrently pursued my research interests in inflammatory and functional bowel disorders, finally to complete my PhD in functional anorectal disorders, from the University of Sydney.
I am currently a senior attending Gastroenterologist at the Rambam Health Care Campus and continue my research as a post-doctorate in the Binshtok lab in Jerusalem. My main research topics include the pathophysiology of senso-motoric abnormalities of the gastrointestinal tract. In my free time I enjoy running, hiking and orienteering, as well as spending time with my beautiful girls and wife.
Bsc in Psychobiology, The hebrew university.