
Dr, Senior Lecturer, emeritus
The pituitary gland plays a central role in the endocrine system; it controls important functions such as growth and metabolism, and regulates hormone secretion from other endocrine glands (e.g., thyroid gland, adrenal gland and gonads). The anterior pituitary (AP) contains five types of hormone secreting cells that secrete at least seven types of hormones. Hypothalamic releasing hormones (RHs) that reach the anterior pituitary via a portal blood system interact with specific receptors on AP cells to either stimulate or inhibit hormone section. AP cells like neurons are excitable cells. A plethora of voltage-gated Na+, K+, and Ca2+ channels generate in AP cells either sporadic or rhythmic electrical activity. The voltage-gated Ca2+ influx (VGCI) that is coupled to this electrical activity plays an essential role in regulating the secretion of AP hormones. It is therefore of interest to investigate the molecular and biophysical properties of Ca2+ channels in anterior pituitary cells. In our experiments we investigated the properties of Ca2+ channels in two populations of AP cells; growth hormone (GH) secreting cells known as somatotrophs and Prolactin secreting cells known as lactotrophs. These AP cell populations were isolated using “density gradient centrifugation”.
The pituitary gland plays a central role in the endocrine system; it controls important functions such as growth and metabolism, and regulates hormone secretion from other endocrine glands (e.g., thyroid gland, adrenal gland and gonads). The anterior pituitary (AP) contains five types of hormone secreting cells that secrete at least seven types of hormones. Hypothalamic releasing hormones (RHs) that reach the anterior pituitary via a portal blood system interact with specific receptors on AP cells to either stimulate or inhibit hormone section. AP cells like neurons are excitable cells. A plethora of voltage-gated Na+, K+, and Ca2+ channels generate in AP cells either sporadic or rhythmic electrical activity. The voltage-gated Ca2+ influx (VGCI) that is coupled to this electrical activity plays an essential role in regulating the secretion of AP hormones. It is therefore of interest to investigate the molecular and biophysical properties of Ca2+ channels in anterior pituitary cells. In our experiments we investigated the properties of Ca2+ channels in two populations of AP cells; growth hormone (GH) secreting cells known as somatotrophs and Prolactin secreting cells known as lactotrophs. These AP cell populations were isolated using “density gradient centrifugation”.
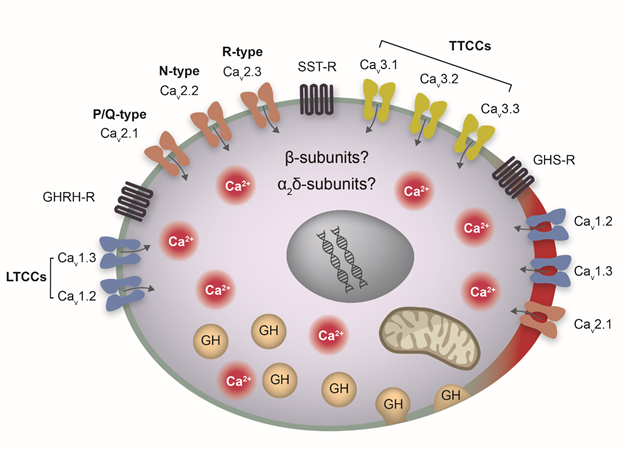
We demonstrated that the VGCI in AP somatotrophs is carried through five isoforms of voltage-gated Ca2+channels (Cav1.2, Cav1.3, Cav2.1, Cav2.2 and Cav2.3), and that these five Ca2+channels are unevenly distributed, among raft and non-raft membrane domains, on the surface membrane of somatotrophs (ref 1,3, Figure 1). We also demonstrated that Ca2+ influx through each of these five Ca2+ channels could regulate GH secretion and that L-type Ca2+ channels (Cav1.2, Cav1.3) contribute the major component to both VGCI and GH secretion (ref 1, 2, Figure 2). These findings pave the way for several interesting questions regarding the potential involvement of Ca2+ channels in GH secretion disorders and regarding their usage as drug targets in the treatment of GH secretion disorders such as Acromegaly and Gigantism (ref 1). An increasing number of studies in recent years have linked genetic missense mutations in L-type Ca2+ channels (Cav1.2, Cav1.3) to diseases of the human cardiovascular, nervous and endocrine systems. These disease-associated genetic mutations occur at homologous functional positions. Thus, it is plausible that similar homologous missense mutations in somatotrophs (in Cav1.2, Cav1.3) can cause abnormal GH secretion (ref 1). This can be further investigated by genetic screening for mutations in Ca2+ channels in human pituitaries, combined with the usage of Cav1.2 and Cav1.3 knockout model mice.
Perturbations in the lipid microenvironment of Ca2+ channels in AP cells that were caused either by insertion of cone shaped lipids into their surrounding membrane (ref 4), or by depletion of cholesterol in their surrounding membrane (ref 2,3), reduced Ca2+ influx and altered Ca2+ channel gating. These findings strongly suggest that the lipid microenvironment plays a critical role in shaping the functional properties of Ca2+ channels in AP cells. This opens questions regarding the physiological significance of this Ca2+ channel sensitivity to lipid stress. One interesting possibility is that Ca2+ channels that are localized in the vicinity to GH exocytotic sites are exposed to massive changes in their lipid microenvironment during the exocytotic event (fusion and hormone release). These massive changes are expected to regulate Ca2+ influx, thus providing a novel autoregulatory mechanism for GH release. This opens interesting questions regarding the actual distance between Ca2+ channels and exocytotic sites in AP cells and regarding their actual quantitative sensitivity to lipid stress.
The cartoon summarizes our current knowledge regarding the Ca2+ channel molecular machinery in rat pituitary somatotrophs. Illustrated are four types of HVA Ca2+ channels, LTCCs (Cav1.2, Cav1.3), P/Q-type (Cav2.1), N-type (Cav2.2) and R-type(Cav2.3). The HVA isoforms Cav1.2, Cav1.3 and Cav2.1 predominantly localize in lipid rafts (illustrated as the thick and red part of membrane). Additionally, the cartoon illustrates three types of TTCCs, Cav3.1 Cav3.2 Cav3.3, and three receptor types that regulate GH secretion, GHRH-R, SST-R and GHS-R (see text). LTCCs, L-type Ca2+ channels; TTCCs, T-type Ca2+ channels; HVA, high voltage-activated.
The Histogram compares between the effects of Ca2+ channel blockers on HVA Ba2+ influx and on “stimulated” GH secretion. The effects of Nifedipine (10 μM), ω-agatoxin-IVA (250 nM) and SNX-482 (30 nM) on L-, PQ- and R-type Ba2+ currents, respectively, were not significantly different from their effects on “stimulated” GH secretion. Yet, the effect of ω- conotoxin GVIA (2 μΜ) on N- type Ba2+ currents was significantly smaller than the effect on “stimulated” GH secretion. Nevertheless, the overall similarity between the effects of CCBs on HVA Ba2+ influx and on “stimulated” GH secretion demonstrates that each one of the four HVA Ca2+ channels in somatotrophs can regulate GH secretion.
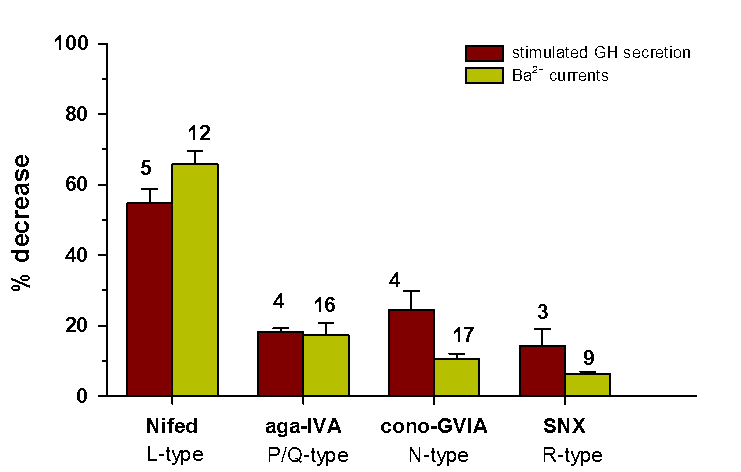
Nussinovitch I. (2018). Ca2+ Channels in Anterior Pituitary Somatotrophs: A Therapeutic Perspective. Endocrinology 159(12), 4043–4055.
Ca2+ influx through voltage-gated Ca2+ channels (VGCCs) plays a key role in GH secretion. In this review, we summarize the current state of knowledge regarding the physiology and molecular machinery of VGCCs in pituitary somatotrophs. We next discuss the possible involvement of Ca2+ channelopathies in pituitary disease and the potential use of Ca2+ channel blockers to treat pituitary disease. Various types of VGCCs exist in pituitary cells. However, because L-type Ca2+ channels (LTCCs) contribute the major component to Ca2+ influx in somatotrophs, lactotrophs, and corticotrophs, we focused on these channels. An increasing number of studies in recent years have linked genetic missense mutations in LTCCs to diseases of the human cardiovascular, nervous, and endocrine systems. These disease-associated genetic mutations occur at homologous functional positions (activation gates) in LTCCs. Thus, it is plausible that similar homologous missense mutations in pituitary LTCCs can cause abnormal hormone secretion and underlying pituitary disorders. The existence of LTCCs in pituitary cells opens questions about their sensitivity to dihydropyridines, a group of selective LTCC blockers. The dihydropyridine sensitivity of pituitary cells, as with any other excitable cell, depends primarily on two parameters: the pattern of their electrical activity and the dihydropyridine sensitivity of their LTCC isoforms. These two parameters are discussed in detail in relation to somatotrophs. These discussions are also relevant to lactotrophs and corticotrophs. High dihydropyridine sensitivity may facilitate their use as drugs to treat pituitary oversecretion disorders such as acromegaly, hyperprolactinemia, and Cushing disease.
Sosial E. and Nussinovitch I. (2015). Multiple Ca2+ Channel-Dependent Components in Growth Hormone Secretion from Rat Anterior Pituitary Somatotrophs. J Neuroendocrinology, 27, 166–176.
The involvement of L-type Ca2+ channels in both ‘basal’ and ‘stimulated’ growth hormone (GH) secretion is well established; however, knowledge regarding the involvement of non-L-type Ca2+ channels is lacking. We investigated whether non-L-type Ca2+ channels regulate GH secretion from anterior pituitary (AP) cells. To this end, GH secretion was monitored from dissociated AP cells, which were incubated for 15 min with 2 mm K+ (‘basal’ secretion) or 60 mm K+ (‘stimulated’ secretion). The role of non-L-type Ca2+ influx was investigated using specific channel blockers, including ω-agatoxin-IVA, ω-conotoxin GVIA or SNX-482, to block P/Q-, N- or R-type Ca2+ channels, respectively. Our results demonstrate that P/Q-, N- and R-type Ca2+ channels contributed 21.2 ± 1.9%, 20.2 ± 7.6% and 11.4 ± 1.8%, respectively, to ‘basal’ GH secretion and 18.3 ± 1.0%, 24.4 ± 5.4% and 14.2 ± 4.8%, respectively, to ‘stimulated’ GH secretion. After treatment with a ‘cocktail’ that comprised the previously described non-L-type blockers, non-L-type Ca2+ channels contributed 50.9 ± 0.4% and 45.5 ± 2.0% to ‘basal’ and ‘stimulated’ GH secretion, respectively. Similarly, based on the effects of nifedipine (10 μM), L-type Ca2+ channels contributed 34.2 ± 3.7% and 54.7 ± 4.1% to ‘basal’ and ‘stimulated’ GH secretion, respectively. Interestingly, the relative contributions of L-type/non-L-type Ca2+ channels to ‘stimulated’ GH secretion were well correlated with the relative contributions of L-type/non-L-type Ca2+ channels to voltage-gated Ca2+ influx in AP cells. Finally, we demonstrated that compartmentalisation of Ca2+ channels is important for GH secretion. Lipid raft disruption (methyl-β-cyclodextrin, 10 mm) abrogated the compartmentalisation of Ca2+ channels and substantially reduced ‘basal’ and ‘stimulated’ GH secretion by 43.2 ± 3.4% and 58.4 ± 4.0%, respectively. In summary, we have demonstrated that multiple Ca2+ channel-dependent pathways regulate GH secretion. The proper function of these pathways depends on their compartmentalisation within AP cell membranes.
Tzour A, Sosial E, Meir T, Canello T, Naveh-Many T, Gabizon R and Nussinovitch I (2013). Multiple pathways for high voltage-activated Ca2+ influx in anterior pituitary lactotrophs and somatotrophs. J Neuroendocrinology 25, 76-86.
The present study demonstrates that a significant proportion of high voltage-activated (HVA) Ca(2+) influx in native rat anterior pituitary cells is carried through non-L-type Ca(2+) channels. Using whole-cell patch-clamp recordings and specific Ca(2+) channel toxin blockers, we show that approximately 35% of the HVA Ca(2+) influx in somatotrophs and lactotrophs is carried through Ca(v) 2.1, Ca(v) 2.2 and Ca(v) 2.3 channels, and that somatotrophs and lactotrophs share similar proportions of these non-L-type Ca(2+) channels. Furthermore, experiments on mixed populations of native anterior pituitary cells revealed that the fraction of HVA Ca(2+) influx carried through these non-L-type Ca(2+) channels might even be higher (approximately 46%), suggesting that non-L-type channels exist in the majority of native anterior pituitary cells. Using western blotting, immunoblots for α(1C) , α(1D) , α(1A) , α(1B) and α(1E) Ca(2+) channel subunits were identified in native rat anterior pituitary cells. Additionally, using reverse transcriptase-polymerase chain reaction, cDNA transcripts for α(1C) , α(1D) , α(1A) and α(1B) Ca(2+) channel subunits were identified. Transcripts for α(1E) were nonspecific and transcripts for α(1S) were not detected at all (control). Taken together, these results clearly demonstrate the existence of multiple HVA Ca(2+) channels in the membrane of rat native anterior pituitary cells. Whether these channels are segregated among different membrane compartments was investigated further in flotation assays, demonstrating that Ca(v) 2.1, Ca(v) 1.2 and caveolin-1 were mostly localised in light fractions of Nycodenz gradients (i.e. in lipid raft domains). Ca(v) 1.3 channels were distributed among both light and heavy fractions of the gradients (i.e. among raft and nonraft domains), whereas Ca(v) 2.2 and Ca(v) 2.3 channels were distributed mostly among nonraft domains. In summary, in the present study, we demonstrate multiple pathways for HVA Ca(2+) influx through L-type and non-L-type Ca(2+) channels in the membrane of native anterior pituitary cells. The compartmentalisation of these channels among raft and nonraft membrane domains might be essential for their proper regulation by separate receptors and signalling pathways.
Ben-Zeev G, Telias M and Nussinovitch I (2010). Lysophospholipids modulate voltage-gated Ca2+ channel currents in pituitary cells; effects of lipid stress. Cell Calcium 47: 514-524.
Voltage-gated calcium channels (VGCCs) are osmosensitive. The hypothesis that this property of VGCCs stems from their susceptibility to alterations in the mechanical properties of the bilayer was tested on VGCCs in pituitary cells using cone-shaped lysophospholipids (LPLs) to perturb bilayer lipid stress. LPLs of different head group size and charge were used: lysophosphatidylcholine (LPC), lysophosphatidylinositol (LPI), lysophosphatidylserine (LPS) and lysophosphatidylethanolamine (LPE). Phosphatidylcholine (PC) and LPC (C6:0) were used as controls. We show that partition of both LPC and LPI into the membrane of pituitary cells suppressed L-type calcium channel currents (IL). This suppression of IL was slow in onset, reversible upon washout with BSA and associated with a depolarizing shift in activation (∼8 mV). In contrast to these effects of LPC and LPI on IL, LPS, LPE, PC and LPC (C6:0) exerted minimal or insignificant effects. This difference may be attributed to the prominent conical shape of LPC and LPI compared to the shapes of LPS and LPE (which have smaller headgroups), and to PC (which is cylindrical). The similar effects of LPC and LPI on IL, despite differences in the structure and charge of their headgroups suggest a common lipid stress dependent mechanism in their action on VGCCs.
I was born in 1948 and grew up in the town Petah Tikva, Israel. Shortly before the end of my army service (as a Lieutenant) I was injured (1968). After a long recovery period, I started my studies in Jerusalem at the Hebrew University. I completed my PhD thesis in the department of physiology (1983) and afterwards moved (with my family) for a three years’ post-doctoral training in Denver, Colorado USA. In 1988 I started my lab in the department of Anatomy, studying properties of Ca2+ channels and their regulatory role in hormone secretion in the anterior pituitary gland. At the present time, after retirement in 2016, I still keep my interests in Ca2+ channels and their role in hormone secretion. Additionally, I continue my teaching at the medical school.