
Professor
In our lab we study basic cellular mechanisms of neuronal function in mammalian cortical structures. Our main focus is on mechanisms that underlie epilepsy, a common neurological disorder (0.5-1% world-wide), characterized by recurrent seizures. A seizure is a sudden interruption in normal brain function during which a large population of neurons fires repetitively and in high synchrony. A cortical structure having a low threshold for seizure generation is the hippocampus. Even when cut into thin slices and maintained in a dish in artificial physiological conditions, rat hippocampal tissue readily can be induced to generate seizure-like discharges. This provides us with an experimentally advantageous model for studying the cellular and molecular mechanisms of epilepsy.
Our scientific research focuses on the mechanisms controlling the intrinsic excitability of nerve cells (neurons) in the hippocampus – a brain structure implicated in learning and memory and in numerous neurological and neurodegenerative diseases. In particular, we study how the expression and function of membrane ion channels and ion pumps, which determine the firing properties of principal neurons, are altered in hippocampal neurons of epileptic animals.
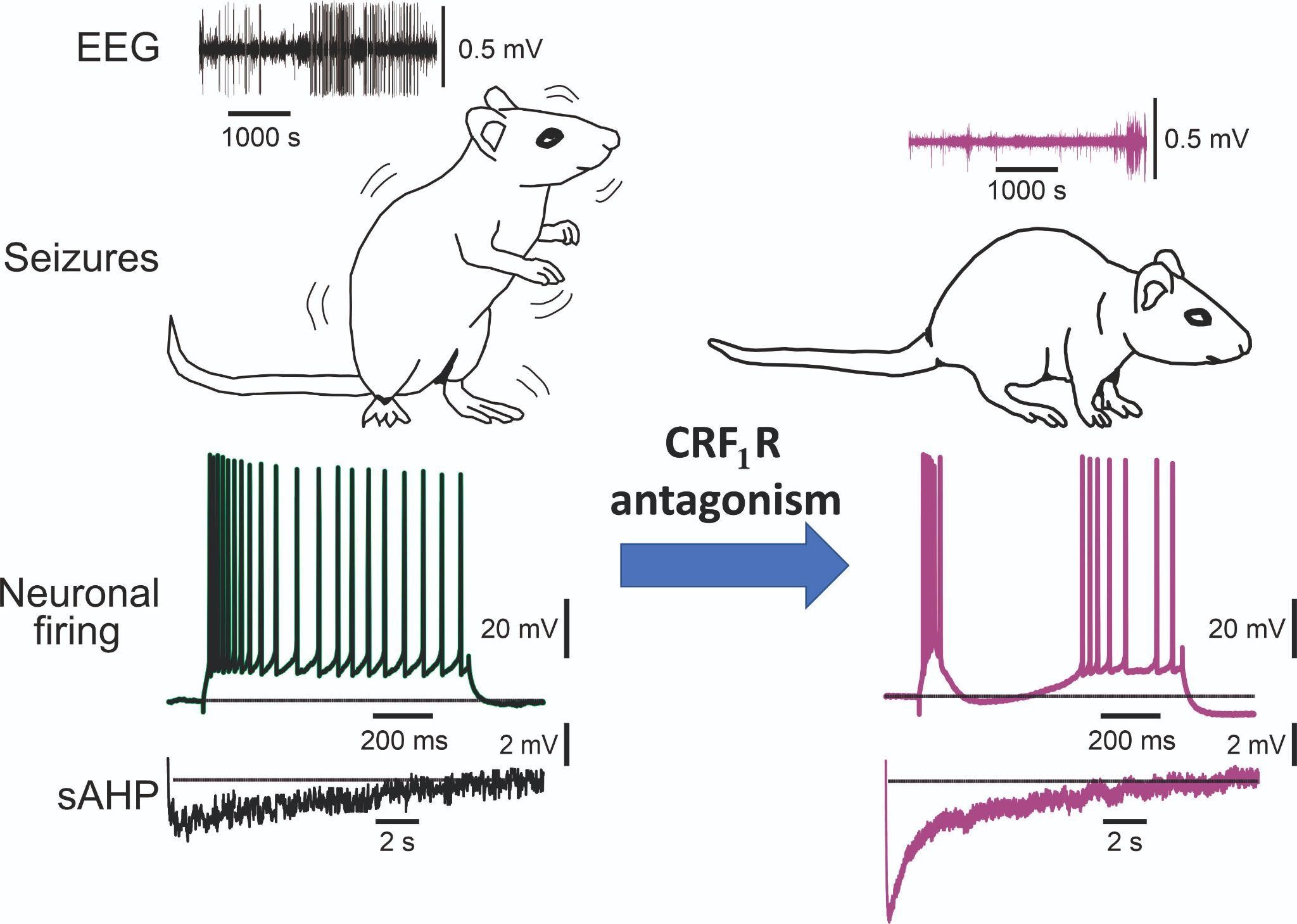
In an experimental rat model of “acquired epilepsy” (chronic epilepsy secondary to a brain insult), we recently found that hippocampal pyramidal cells become intrinsically hyperexcitable. This alteration is due predominantly to a protein kinase A (PKA)-mediated of a ubiquitous class of potassium ion channels, KCa3.1, whose main function is to dampen neuronal excitability. Most importantly, this “acquired channelopathy” can be acutely reversed by PKA inhibitors, leading to recovery of function and normalization of neuronal excitability.
More recently we found in this model that the PKA signaling causing KCa3.1 downregulation is due to enhanced protein expression of corticotropin releasing factor (CRF) and of its type 1 receptor (CRF1R) in the hippocampus. Congruently, acute application of selective CRF1R antagonists restored KCa3.1 channel activity, thereby restoring KCa3.1 activity and normalizing intrinsic neuronal excitability. Moreover, we found that even a single injection of an CRF1R antagonist to chronically epileptic animals markedly decreases the frequency of electrographic seizures in all treated animals for several hours after treatment. We now attempt to translate these basic findings to novel antiepileptic therapies.
ISF
DFG
Mohan S, Tiwari MN, Stanojević M, Biala Y, Yaari Y. (2021). Muscarinic regulation of the neuronal Na+/K+-ATPase in rat hippocampus. Journal of Physiology 599:3735-3754.
Stimulation of postsynaptic muscarinic receptors was shown to excite principal hippocampal neurons by modulating several membrane ion conductances. We show here that activation of postsynaptic muscarinic receptors also causes neuronal excitation by inhibiting Na+ /K+ -ATPase activity. Muscarinic Na+ /K+ -ATPase inhibition is mediated by two separate signalling pathways that lead downstream to enhanced Na+ /K+ -ATPase phosphorylation by activating protein kinase C and protein kinase G. Muscarinic excitation through Na+ /K+ -ATPase inhibition is probably involved in cholinergic modulation of hippocampal activity and may turn out to be a widespread mechanism of neuronal excitation in the brain.
Tiwari MN, Mohan S, Biala Y, Yaari Y. (2019). Protein kinase A-mediated suppression of the slow afterhyperpolarizing KCa3.1 current in temporal lobe epilepsy. Journal of Neuroscience, 39:9914–9926.
Epilepsy, a common neurological disorder, often develops following a brain insult. Identifying key molecular and cellular mechanisms underlying acquired epilepsy is critical for developing effective antiepileptic therapies. In an experimental model of acquired epilepsy, we show that principal hippocampal neurons become intrinsically hyperexcitable. This alteration is due predominantly to the downregulation of a ubiquitous class of potassium ion channels, KCa3.1, whose main function is to dampen neuronal excitability. KCa3.1 downregulation is mediated by the cAMP-dependent protein kinase A (PKA) signaling pathway. Most importantly, it can be acutely reversed by PKA inhibitors, leading to recovery of KCa3.1 function and normalization of neuronal excitability. The discovery of this novel epileptogenic mechanism hopefully will facilitate the development of more efficient pharmacotherapy for acquired epilepsy.
Mohan S, Tiwari MN, Biala Y, Yaari Y. (2019). Regulation of neuronal Na+/K+-ATPase by specific protein kinases and protein phosphatases. Journal of Neuroscience, 39:5440 –5451.
The Na+/K+-ATPase (NKA), known as the “Na+ pump,” is a ubiquitous membrane-bound enzyme responsible for generating and maintaining the Na+ and K+ electrochemical gradients across the plasma membrane of living cells. In neurons, as in most types of cells, the NKA generates the negative resting membrane potential, which is the basis for almost all aspects of cellular function. Here we used an electrophysiological approach to monitor physiological NKA transport activity in single hippocampal pyramidal cells in situ. We have found that neuronal NKA activity is oppositely regulated by phosphorylation and dephosphorylation, and we have identified the main protein kinases and phosphatases mediating this regulation. This fundamental form of NKA regulation likely plays a role in multiple brain functions.
Tiwari MN, Mohan S, Biala Y, Yaari Y. (2018). Differential contributions of Ca2+-activated K+ channels and Na+/K+-ATPases to the generation of the slow afterhyperpolarization in CA1 pyramidal cells. Hippocampus 28:338-357.
In many types of CNS neurons, repetitive spiking produces a slow afterhyperpolarization (sAHP), providing sustained, intrinsically generated negative feedback to neuronal excitation. Changes in the sAHP have been implicated in learning behaviors, in cognitive decline in aging, and in epileptogenesis. Despite its importance in brain function, the mechanisms generating the sAHP are still controversial. Here we have addressed the roles of M‐type K+ current (I M), Ca2+‐gated K+ currents (I Ca(K)’s) and Na+/K+‐ATPases (NKAs) current to sAHP generation in adult rat CA1 pyramidal cells maintained at near‐physiological temperature (35 °C). No evidence for I M contribution to the sAHP was found in these neurons. Both I Ca(K)’s and NKA current contributed to sAHP generation, the latter being the predominant generator of the sAHP, particularly when evoked with short trains of spikes. Of the different NKA isoenzymes, α1‐NKA played the key role, endowing the sAHP a steep voltage‐dependence. Thus normal and pathological changes in α1‐NKA expression or function may affect cognitive processes by modulating the inhibitory efficacy of the sAHP.
Tamir I, Daninos M, Yaari Y. (2017). Plasticity of intrinsic firing response gain in principal hippocampal neurons following pilocarpine-induced status epilepticus. Neuroscience 357:325-337.
Here we show that pilocarpine-SE causes multiplication of the firing response gain in the three principal neurons in the hippocampal trisynaptic pathway. This alteration undoubtedly would contribute to hippocampal hyperexcitability in SE-induced TLE.
Tzour A, Biala Y, Lev S, Yaari Y, Binshtok AM. (2017). KV7/M channels as targets for inflammation-induced neuronal hyperexcitability. Journal of Physiology 595:713-738.
Acute brain insults and many chronic brain diseases manifest an innate inflammatory response. The hallmark of this response is glia activation, which promotes repair of damaged tissue, but also induces structural and functional changes that may lead to an increase in neuronal excitability. We have investigated the mechanisms involved in the modulation of neuronal activity by acute inflammation. Initiating inflammatory responses in hippocampal tissue rapidly led to neuronal depolarization and repetitive firing even in the absence of active synaptic transmission. This action was mediated by a complex metabotropic purinergic and glutamatergic glia‐to‐neuron signalling cascade, leading to the blockade of neuronal KV7/M channels by Ca2+ released from internal stores. These channels generate the low voltage‐activating, non‐inactivating M‐type K+ current (M‐current) that controls intrinsic neuronal excitability, and its inhibition was the predominant cause of the inflammation‐induced hyperexcitability. Our discovery that the ubiquitous KV7/M channels are the downstream target of the inflammation‐induced cascade, has far reaching implications for the understanding and treatment of many acute and chronic brain disorders.
Website designed by toornet