
Professor
My investigations have created a new field of research. I co-discovered a new type of ion channel, which I designated the Transient Receptor Potential or in short the TRP channel, as a result of my studies on phototransduction and vision in fruit flies (Drosophila melanogaster). I investigated the biophysical and biochemical properties of TRP channels in fruit fly eyes and identified together with the late Prof. Zvi Selinger phospholipase C as a crucial component of the Drosophila TRP signaling pathway. The principles that we found are common to numerous sensory (e.g. temperature, pain and taste sensations) and motor systems (properties of Purkinje neurons in the cerebellum), thus laying the foundation for the study of molecules that underlie mechanisms of TRP and pain, originally initiated by Prof. David Julius. Today we have extended our research to the first identified loss of function mutation in human TRPV1 channel that governs at least several of the human body’s regulatory processes related to inflammatory pain and to noxious heat detection. Our major aim is to decipher the critical roles of TRP channels in invertebrate vision and of TRPV1 in human somatosensory physiology
The Drosophila TRP channels are the founding members of a large superfamily of channel proteins, which is conserved through evolution from fly to human and have prime importance in many of our body functions. These functions include control of body temperature, pain, perception of taste, important functions of the kidney, the intestine, the lungs, the nervous system and more. Malfunction of TRP channels is involved in many human diseases. The long-term goal of this project is to unravel the still unknown physiological activation mechanism of the Drosophila light sensitive channels TRP. In the present project we use modulation of membrane sterols, in particular ergosterol, as a new and powerful tool that, so far, has not been used to study the mechanism of Drosophila TRP channels activation in their native environment of the photoreceptor cells. One important tool is to determine whether our observed suppression of the light response by ergosterol sequestration by methyl β cyclodextrin (MβCD) arises from suppression of Phospholipase C (PLC) enzymatic activity that plays a central role in phototransduction. To achieve this aim, we use sensitive fluorescent probes that monitor PLC activity in vivo simultaneously with the response to light, testing the photomechanical model of TRP channel activation (see Fig. 1).
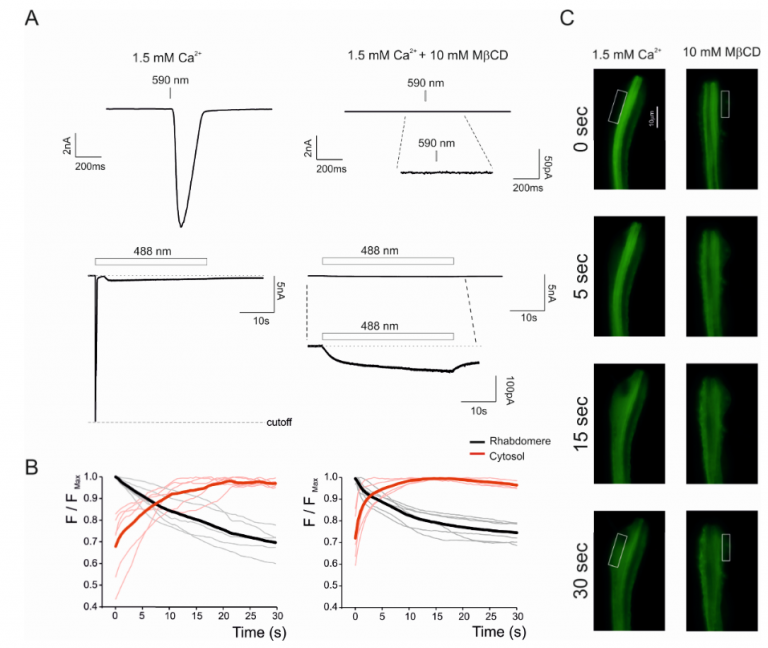
Figure. 1: Translocation of fluorescent TbR323H-YFP probe in dissociated ommatidia. Simultaneous recordings of fluorescent images (C and its quantification in B,) and Light-Induced Current (LIC) by whole cell recordings (A) from initially dark-adapted dissociated ommatidium expressing TbR332H immediately at the onset of blue excitation (t=0) and during 30s blue light pulse in the presence of MβCD (10mM). A sample of fluorescence images in control solution (C left) and in MβCD (C, right) are presented at 0, 5, 15 and 30 s after light onset. Fluorescence was initially strongest in the rhabdomeres, but rapidly translocated to cell body and plasma membrane (white rectangular C). The upper electrophysiological traces show at a faster time scale the LIC in response to much dimmer orange (590nm) light that does not induce fluorescence. The lower trace shows the LIC to intense blue light that was used in the fluorescence measurements. (B) Time course of translocation measured as the normalized ratio of fluorescence (from boxes as in C) in rhabdomere and cytosol (F/FMax). Each thick trace is the mean of measurements from 6 ommatidia. (Graph) Extent of fluorescence change (F/FMax /s) expressed as ratio between F/FMax at time zero and after 30 s illumination. Strikingly, addition of MβCD (right graph, red) induced the fastest kinetics, most likely because suppressing the activity of the highly Ca2+-permeable TRP channel that largely reduced intracellular Ca2+ and hence Ca2+-dependent inhibition of PLC. Very similar acceleration of the translocation was obtained by Hardie and colleagues in the trp;trpl double null mutant in which Ca2+influx via the TRP/TRPL channels was abolished. This similarity obtained by two independent ways of suppressing the TRP/TRPL-dependent currents strongly support the reliability of our measurements. The data shows that PLC hydrolyzing activity is not directly involved in TRP gating.
Membrane cholesterol is known to have profound effects on ion channel activity. In order to examine the possibility of a direct binding-site for cholesterol on solved structure of a TRP channel, the Levitan lab performed docking analysis of cholesterol with (rat) rTRPV1. Since cholesterol has a regulatory role on the rTRPV1 activity, we searched for amino acid residues that interact with cholesterol. Three of the putative cholesterol-interacting residues within the list of stable rTRPV1WT binding clusters were studied in detail. We have applied whole cell patch clamp measurements of mutant TRPV1 expressed transiently in T-REx-293 cells. Our results suggest that cholesterol directly binds the TRPV1 channel and acts as an inhibitor of its activity. Whether this is achieved by a competition with capsaicin, or by stabilization of the closed state of the channel, or by a combination of both, remains to be determined.
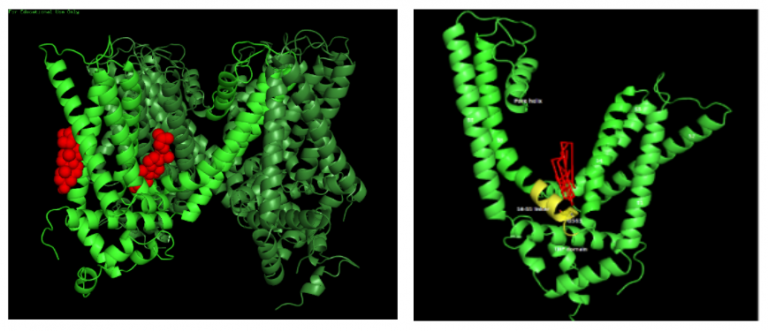
Figure. 2 (left). The rTRPV1WT atomic structure in low resolution as determined by Cryo-EM. Tetramer with two predicted pockets for the binding of cholesterol: One of the channel’s subunits is highlighted with light green. Cholesterol molecules in its two putative binding pockets (red): to the left, an annular position in a cleft between S1 and S2 helices. To the right, a non-annular pocket between two subunits, where the S5 and S6 helices of one subunit are interacting with the S4-S5 linker and the S4 helices of another subunit
Figure 2(right): Docking analysis of cholesterol on rTRPV1 and the predicted binding pose. The figures show the predicted orientation of cholesterol (red) and interacting residues in the transmembrane view of TRPV1 structure showing only one subunit of the channel.
Membrane cholesterol is known to have profound effects on ion channel activity. In order to examine the possibility of a direct binding-site for cholesterol on solved structure of a TRP channel, the Levitan lab performed docking analysis of cholesterol with (rat) rTRPV1. Since cholesterol has a regulatory role on the rTRPV1 activity, we searched for amino acid residues that interact with cholesterol. Three of the putative cholesterol-interacting residues within the list of stable rTRPV1WT binding clusters were studied in detail. We have applied whole cell patch clamp measurements of mutant TRPV1 expressed transiently in T-REx-293 cells. Our results suggest that cholesterol directly binds the TRPV1 channel and acts as an inhibitor of its activity. Whether this is achieved by a competition with capsaicin, or by stabilization of the closed state of the channel, or by a combination of both, remains to be determined.
Figure 3
Small flying organisms face rapid contrast changes that can be mimicked by oscillating light of variable frequencies. Drosophila photoreceptors respond to oscillating light of high frequency (~100 Hz), while the frequency response of human cones is significantly lower (~50 Hz). The detected maximal frequency is modulated by ambient illumination, allowing both high sensitivity and temporal resolution, via an unclear mechanism. In this project we studied the molecular mechanism underlying light-dependent modulations of the Drosophila frequency response to oscillating light. We found that the phosphorylation state of a specific site (Ser936) at the light-activated Transient Receptor Potential (TRP) channel determines the maximal detected frequency of oscillating light. Recently, Huber and colleagues identified light-regulated phosphorylation sites of Drosophila TRP channel using quantitative mass spectrometry (Fig. 4 left). Many of the identified phosphorylation sites displayed enhanced phosphorylation in the light, while a single phosphorylation site, Ser936, was predominantly phosphorylated in the dark and became dephosphorylated upon illumination (Fig. 4 left black circle). To investigate the physiological roles of light-dependent TRP dephosphorylation at the Ser936 site we generated a transgenic fly that expresses a TRP channel in which Serine 936 was exchanged with Alanine (trpS936A). In WT but not in the mutant there was a marked difference between the frequency response of light-adapted and dark-adapted flies at high stimulus frequency. The results indicate that the TRP channel phosphorylation state at the Ser936 is a light dependent adaptable process, which determines the detection limit of oscillating light according to ambient illumination, adjusting dynamic processing of visual information under variable light conditions.
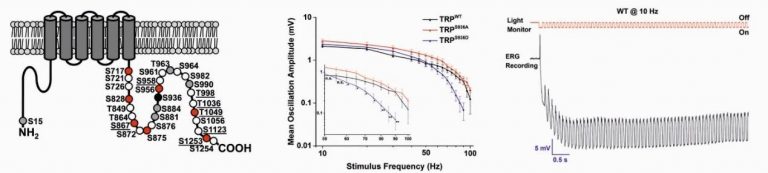
Figure. 4 Left: Structural features of the Drosophila TRP channel showing its phosphorylation sites. The Serine 936 site is marked by black circle. Middle: Frequency response to oscillating light of wild type (trpWT) and two transgenic flies carrying the S936A (trpS936A) and S936D (trpS936D) mutations. Right: A representative example of an ERG response to oscillating light of 10 Hz recorded from a light-adapted WT fly. The initial positive “spike” is the “on” transient coming from the lamina neurons. The upper red trace depicts the light stimulus measured simultaneously by a phototransistor.
Katz, B., Zaguri, R., Edvardson, S., Maayan, C., Elpeleg, O., Lev, S., Davidson, E., Peters, M., Kfir-Erenfeld, S., Berger, E., Ghazalin, S., Binshtok, A.M., and Minke, B. (2022). Nociception and pain in humans lacking functional TRPV1 channel. J Clin. Invest. https://doi.org/10.1172/JCI153558
Brandwine, T. Ifrah R., Bialistoky, T. Zaguri, R. Rhodes-Mordov E., Mizrahi-Meissonnier L., Sharon D., Katanaev V., Gerlitz O and Minke B. (2021) Knockdown of dehydrodolichyl diphosphate synthase (DHDDS) in the Drosophila retina leads to a unique pattern of retinal degeneration. Front Mol Neurosci. 2021 Jul 5;14: 693967. doi: 10.3389/fnmol.2021.693967. eCollection 2021.
Compromising DHDDS in the developing retina, while allowing formation of the retina, resulted in a unique pattern of retinal degeneration, characterized by a dramatic reduction in rhodopsin protein level and an abnormal accumulation of ER membranes in the photoreceptors cells, thus indicating that DHDDS is essential for normal retinal formation.
Voolstra, O., Rhodes-Mordov E., Katz, B., Bartels, J.P., Oberegelsbacher, C., Schotthöfer, S.K., Yasin, B., Tzadok, H., Huber, A., and Minke, B. (2017) The phosphorylation state of the Drosophila TRP channel modulates the frequency response to oscillating light in vivo. Neurosci. 12;37:4213-4224.
Drosophila photoreceptors exhibit high temporal resolution as manifested in frequency response to oscillating light of high frequency (≤∼100 Hz). Light rearing conditions modulate the maximal frequency detected by photoreceptors, thus enabling them to maintain high sensitivity to light and high temporal resolution. However, the precise mechanisms for this process are not fully understood. Here, we show by combination of biochemistry and in vivo electrophysiology that transient receptor potential (TRP) channel dephosphorylation at a specific site is a fast, light-activated and Ca2+-dependent regulatory process. TRP dephosphorylation affects the detection limit of oscillating light according to the adaptation state of the photoreceptor cells by shifting the detection limit to higher frequencies upon light adaptation. This novel mechanism thus adjusts dynamic processing of visual information under varying light conditions.
Devary, O., O. Heichal, A. Blumenfeld, D. Cassel, E. Suss, S. Barash, C. Rubinstein, B. Minke and Z. Selinger. Coupling of photoexcited rhodopsin to inositol phospholipid hydrolysis in fly photoreceptors. Proc. Natl. Acad. Sci. USA. 84: 6939-6943 (1987).
The discovery that the light sensitive TRP channels are activated via the inositol lipid signaling.
Cook B, Bar-Yaacov M, Cohen Ben-Ami H, Goldstein R.E., Paroush Z, Selinger Z and Minke B. (2000). Phospholipase C and termination of G-protein-mediated signalling in vivo, Nature Cell Biology, 2: 296-301 (covered by News & Views).
The discovery that fly PLC functions as a GTPase-Activating-Protein (GAP) in vivo.

PhD student
Studies of TRP channel properties in the Drosophila eye

PhD student
Investigating Functional Properties of TRP Channels

PhD student
Analysis of a human mutation located at the Ankyrin-repeat domain of the TRPV1 channel

Website designed by toornet
Born in Tel-Aviv, Israel, Baruch Minke received his Ph.D. degree in physiology and biophysics from the Hebrew University of Jerusalem. During his postdoctoral training at Purdue University in the USA (during 1973-1975), he has recognized the great power of genetic and molecular genetic and combined this approach to his electrophysiological and biochemical studies of sensory systems in the fruit fly Drosophila. He is a professor at the Faculty of Medicine of the Hebrew University and Hadassah Hospital in Jerusalem and a member of the Edmond and Lily Safra Center for Brain Sciences (ELSC).
He was a visiting research professor for extended periods at the Max-Planck-Institut für Biologische Kybernetik in Tübingen, Germany; at Experimental Ophthalmology, Hospital Cantonal Geneva, Switzerland; at the Institute for Biology, the Technical University RWTH, Aachen, Germany; at the Australian National University, Canberra, Australia; at the Department of Neurosciences, The Johns Hopkins University School of Medicine, Baltimore, USA and at the Department of Biology, the University of California at San Diego (UCSD), USA. He was the head of the Department of Physiology at the Faculty of Medicine of the Hebrew University and Advisor of the Israeli Committee for High Education.
He was a Member of the Editorial Board of the International Journal “Photobiochemistry and Photobiophysics” (Elsevier/North Holland); Member of the Board of Editors of the International Journal “Visual Neuroscience” and Member of the Editorial Board of the International Journal “The European Journal of Physiology/Pflügers Archiv” and Member of the Board of Editors of the International Journal “Scientific Reports”, a journal from Nature Publishing Group.
He is currently Member of the Editorial Board of the International Journal “Cell Calcium” (Elsevier/North Holland) He was the organizer of 4 International conferences. He has been awarded 10 National Institute of Health (NIH, USA) grants as an independent Foreign Investigator (RO1). He received the Senior Fulbright-Hays Program Award (USA), the Bat-Sheva de Rothschild Foundation Award for Excellent research (Jerusalem), the Volkswagenwerk Foundation Grant (Germany) and the Wolf Grant for Excellent Research (Wolf Foundation, Israel), the EMET prize in Life Sciences on Brain Research from the Israel Prime Minister and the Prince of Asturias Award for Science and Technology from Spain.