
Senior Lecturer
We are interested in how animals and humans perceive the visual world and use this perceptual information to guide their behavior. Thus, by studying the structure and function of genetically distinct retinal and brain circuits, we seek to link the processing of brightness, contrast, color, and motion information to behavior.
We use mouse genetic models and combine:
Reconstruction of neural circuits using light and electron microscopy
in vitro and in vivo electrophysiology and functional imaging
Optogenetic and chemogenetic manipulations of neural activity
Behavioral analysis
For human research, we use functional magnetic resonance imaging (fMRI).
These powerful and complementary approaches allow us to reveal the operation of specific neural circuits and their role in shaping the behavior of animals and humans in unprecedented detail.
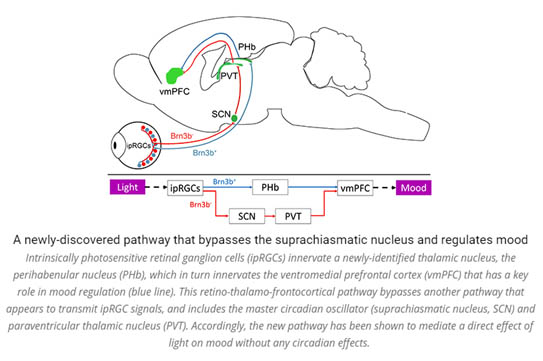
The main pathway through which visual signals are routed to cortex includes the dorsal lateral geniculate nucleus and primary visual cortex. However, recent work has identified in the mouse a new separate pathway. This pathway includes the dorso-thalamic perihabenula and the ventromedial prefrontal cortex which plays a key role in mood regulation. In addition to studying this newly identified pathway in the mouse, our lab uses functional MRI to test whether the new pathway exists also in humans, and if so, whether it contributes to mood regulation.
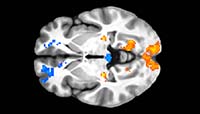
Orientation selective neurons, which respond to edges presented at certain orientations, have been documented in the retina and cortex. However, it is unknown whether cortical orientation selectivity is created de novo in the cortex, or instead derived from processing and integration of orientation-selective signals arriving from the retina, presumably through thalamic and collicular relay neurons. To understand the basis of retinal orientation selectivity and its contribution to cortical orientation selectivity, we study the topographic variation, morphology, physiology, and central projections of orientation-selective retinal ganglion cells.
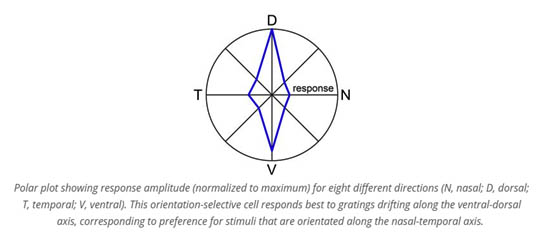
Visual motion tells us how objects are moving in the world, and how we are moving within that world. Our work has recently transformed the understanding of this system’s architecture in the context of the whole animal. We studied how the global geometry of retinal direction selectivity relates to optic flow induced by self-motion. By intensive global mapping using two-photon calcium imaging, electrophysiology, and retrograde tracing, we revealed a surprising spherical geometry of retinal direction selectivity. We identified four subtypes of direction-selective ganglion cells, each of which aligned its directional preferences with optic flow produced by the mouse’s movement along either the body or gravitational axis. This has fascinating implications for all the perceptual and visuomotor functions supported by these cells, including image stabilization, cortical motion perception, and gaze shifts to moving targets. Nowadays, we study how signals from the various subtypes of direction-selective ganglion cells interact in the brain to generate an estimate of the direction of motion and trigger compensatory eye movements. We also study the mechanistic basis of retinal direction selectivity, and specifically, the asymmetry of input to direction-selective retinal ganglion cells from presynaptic cells.
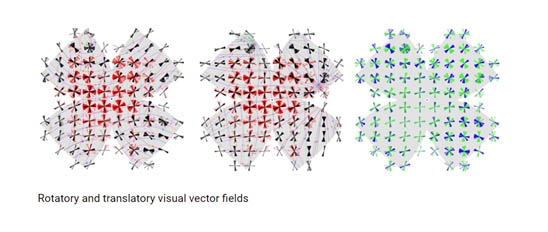
Light has a critical impact on mood disorders in ways we are only beginning to recognize, with the potential for the development of effective, relatively non-invasive therapies. However, until now, surprisingly little has been reported on the mechanisms underlying the effects of light on mood. We characterize a newly discovered pathway that links specialized retinal photoreceptors, the dorsal thalamus, and the prefrontal cortex. This pathway apparently mediates the diverse effects of diffuse light intensity on mood. We dissect the synaptic input properties, behavioral output, and mechanistic basis of this new pathway, with special emphasis on its contribution to the pathophysiology of depression. To this end, we combine complementary behavioral, electrophysiological, functional imaging, and genetic manipulation approaches. This study, we hope, will pave the way toward a new understanding of the effect of light on mood, which can have implications both for research and in the clinic.
The mammalian retina maintains high sensitivity over an extraordinary range of luminance levels, ranging from starlight to bright sunlight. This is achieved by switching between the rod and cone systems, and within each system, by employing light adaptation mechanisms that preserve a contrast-invariant response. While dopamine is known to modulate retinal network activity in proportion to luminance, the luminance-dependent signals and the circuits that transmit them to elicit light adaptation, remain elusive. Our work identified a family of luxotonic amacrine cells in which activity increases with luminance level and are poised to modulate retinal function in a luminance-dependent manner. We determine the kinetics of the synaptic input to luxotonic amacrine cells, identify their pre- and postsynaptic partners, and test their role in retinal light adaptation. Implementing optogenetics, chemogenetics, functional imaging, whole-cell electrophysiology, and serial section electron microscopy, this study will help further our understanding of a fundamental adaptative mechanism in a key sensory modality.

Israel Science Foundation (ISF)
United States – Israel Binational Science Foundation (BSF)
National Institute for Psychobiology in Israel (NIPI)
Brain & Behavior Research Foundation (BBRF)
Laniado, D. D., Maron, Y., Gemmer, J. A., & Sabbah, S. Spherical code of retinal orientation-selectivity enables decoding in ensembled and retinotopic operation. (2025) Cell Reports
https://doi.org/10.1016/j.celrep.2025.115373
Selectivity to orientations of edges is seen at the earliest stages of visual processing in retinal orientation-selective ganglion cells (OSGCs), which are thought to prefer vertical or horizontal orientation. However, because stationary edges are projected on the hemispherical retina as lines of longitude or latitude, how edge orientation is encoded and decoded by the brain is unknown. Here, by mapping the orientation selectivity (OS) of thousands of OSGCs at known retinal locations in mice, we identify three OSGC types whose preferences match two longitudinal fields and a fourth type matching two latitudinal fields, with the members of each field pair being non-orthogonal. A geometric decoder reveals that two OS sensors yield optimal orientation decoding when approaching the deviation from orthogonality we observe for OSGC field pairs. Retinotopically organized decoding generates type-specific variation in decoding efficiency across the visual field. OS tuning is greater in the dorsal retina, possibly reflecting an evolutionary adaptation to an environmental gradient of edges.
Zangen, E., Hadar, S., Lawrence, C. et al. Prefrontal cortex neurons encode ambient light intensity differentially across regions and layers. (2024) Nat Commun. https://doi.org/10.1038/s41467-024-49794-w
While light can affect emotional and cognitive processes of the medial prefrontal cortex (mPFC), no light-encoding was hitherto identified in this region. Here, extracellular recordings in awake mice revealed that over half of studied mPFC neurons showed photosensitivity, that was diminished by inhibition of intrinsically photosensitive retinal ganglion cells (ipRGCs), or of the upstream thalamic perihabenular nucleus (PHb). In 15% of mPFC photosensitive neurons, firing rate changed monotonically along light-intensity steps and gradients. These light-intensity-encoding neurons comprised four types, two enhancing and two suppressing their firing rate with increased light intensity. Similar types were identified in the PHb, where they exhibited shorter latency and increased sensitivity. Light suppressed prelimbic activity but boosted infralimbic activity, mirroring the regions’ contrasting roles in fear-conditioning, drug-seeking, and anxiety. We posit that prefrontal photosensitivity represents a substrate of light-susceptible, mPFC-mediated functions, which could be ultimately studied as a therapeutical target in psychiatric and addiction disorders.
Shai Sabbah, Michael S. Worden, Dimitrios D. Laniado, David M. Berson and Jerome N. Sanes. (2022). Luxotonic signals in human prefrontal cortex as a possible substrate for effects of light on mood and cognition. PNAS doi/10.1073/pnas.2118192119
Humans sense changes in ambient illumination, thus luxotonic properties, unrelated to form vision, and these changes influence a wide range of functions, including circadian rhythms, visual reflexes, mood, and likely cognitive processing. While image-forming pathways in the primate brain detect minute changes in illumination, it remains unclear how light-intensity signals reach and become processed in brain structures involved in basic moods and their dysfunction, pathways that likely derive from intrinsically photosensitive retinal ganglion cells. Here, we show that prefrontal regions in the human brain have luxotonic signals. These signals have properties similar to intrinsically photosensitive retinal ganglion cells, and they may underlie light-intensity effects on complex behaviors.
Sabbah S, Gemmer JA, Bhatia-Lin A, Manoff G, Castro G, Siegel JK, Jeffery N and Berson DM. (2017). A retinal code for motion along the gravitational and body axes. Nature (full-length article) 546:492-497.
We studied how the global geometry of retinal direction selectivity relates to optic flow induced by self-motion. By intensive global mapping using two-photon calcium imaging, electrophysiology, and retrograde tracing, we revealed a surprising spherical geometry of retinal direction selectivity. We identified four subtypes of direction-selective ganglion cells, each of which aligned its directional preferences with optic flow produced by the mouse’s movement along either the body or gravitational axis. This has fascinating implications for all the perceptual and visuomotor functions supported by these cells, including image stabilization, cortical motion perception, and gaze shifts to moving targets.
Stabio ME, Sabbah S, Quattrochi L, Ilardi MC, Fogerson M, Leyrer M, Renna JM, Kim MT, Kim I, Schiel M, Briggman KL, Berson DM. (2018). The M5 cell: A color-opponent intrinsically photosensitive retinal ganglion cell. Neuron 97, 150-163.
We documented the fifth member (M5) of the intrinsically photosensitive retinal ganglion cells (ipRGCs) family. Our results demonstrated that this is the only known cell in the mouse retina to blend melanopsin-based intrinsic photosensitivity, a critical property for non-image forming visual behaviors, with chromatically opponent cone inputs. Thus, this cell may provide the brain with information about both environmental light intensity and color.
Sabbah S, Berg D, Papendorp C#, Briggman KL and Berson DM*. (2017). A Cre mouse line for probing irradiance- and direction-encoding retinal networks. eNeuro doi:10.1523/eneuro.0065-17.2017.
We showed that the Rbp4-Cre transgenic mouse marks and permits selective manipulation of a specific class of retinal interneurons (amacrine cells) that are electrically coupled to, and receive intensity-encoding signals from, ipRGCs. Thus, this network represents a mechanism for ipRGC intensity signals to modulate the responses of retinal neurons, perhaps as a component of light adaptation.
We are currently recruiting highly motivated candidates for M.Sc. and Ph.D. studies
We study
neural circuits that underlie key visual functions in the retina and brain, including light adaptation and direction – and orientation-selectivity. We also study non-image-forming photoreception and the role of diverse brain pathways in mediating the effect of abnormal lighting on mood.
Our research
integrates an unparalleled array of cutting-edge techniques:
Successful candidates will show solid communication skills in English, ability to work both independently and as part of a research team, strong scientific motivation, and skill in data processing. Experience in one or more of the following would be an advantage: intracranial surgeries, optogenetics, animal behavioral analysis, two-photon imaging, whole-cell and in vivo electrophysiology, and Matlab or python programing.
An application package, including a motivation letter, curriculum vitae, and names of 2-3 referees, should be sent to Dr. Shai Sabbah (shai.sabbah@mail.huji.ac.il).
Born in Dimona in southern Israel, I started my academic journey with an undergraduate degree in biology from the Technion – Israel Institute of Technology, Haifa. I went on to the Hebrew University for my master’s degree under the supervision of Prof. Nadav Shashar in the Department of Evolutions, Systematics and Ecology. For my doctorate, I travelled to Queen’s University in Kingston, Ontario, Canada where, under the supervision of Prof. Craig Hawryshyn, I characterized the underwater light environment and explored how evolution has shaped the properties of cone photoreceptors in the vertebrate retina. I employed electrophysiological techniques to assess functional diversity in the visual systems of fish and probed how the visual system changes with development and across habitats. I then took on a postdoctoral research position in the Department of Neuroscience at Brown University, Providence, RI, under the mentorship of Prof. David Berson. My research there focused on (1) image stabilization and the tuning of direction selective cells in the retina, and (2) mechanisms of luminance coding in the retina. In 2018, I returned to the Hebrew University and established my laboratory for Visual Neuroscience, Mood, and Behavior at the Faculty of Medicine.