
Professor
Motor control has been assigned to neural circuits located within the spinal cord. At the core of the spinal motor system are sets of local interneurons (INs) that are wired in neuronal circuits capable of controlling the activity and output of spinal motor neurons. Interneurons differ from each other by their type of afferent input, cell body positioning along the body axis, axonal trajectory and their axonal targets. Each IN expresses a defined set of transcription factors (transcriptional code) that subsequently determine its axonal trajectories and target selection. A specific axonal pathway of a neuron, governed by a transcriptional code, is manifested by the expression of receptors for guidance molecules that discern the guidance cues en route and at their putative target.
Studies in our lab are aimed toward decoding the wiring of spinal neuronal circuits and understanding the role of interneurons in motor control. Specifically: defining the axonal trajectories of spinal IN and their neuronal circuit; elucidating the transcriptional code that governs the axonal patterning of INs; determining the role of guidance molecules at axonal choice points; characterizing neuronal receptors for positional guidance cues that control axonal choice; ascertaining the role of defined IN populations in simple motor behaviors; and revealing the species-specific networks that evolved to support maneuvering in various environmental milieus.
The functioning of a living organism relies on its ability to sense the environment and to respond accordingly. The nervous system senses and processes divergent external stimuli and reacts via activation of specific motor tasks. The spinal cord has a profound role in the sensorimotor system: 1) it serves as a relay station that ascends to the brain peripheral sensory information and receives descending cortical motor commands; 2) has an autonomous role in maintaining body posture; 3) controls the coordinated activity of antagonistic muscles and the bilateral limbs musculature; 4) provides the cerebellum sensory and motor feedback information required for stable and coordinated locomotion. The diversity of neurons within the spinal cord and their circuitry are crucial for attaining these functions. the studies in our laboratory are aimed at elucidating the ontogeny and phylogeny of spinal interneurons (INs), their circuitry, and the genetic and molecular pathways that instruct their wiring and function.
Interneurons within the spinal cord and the hindbrain are subdivided into cardinal subtypes based on their transcriptional code. Interneurons differ by their type of afferent input, cell body positioning along the body axes, axonal trajectory, and axonal targets. A specific axonal pathway of a neuron is governed by a transcriptional code, manifested by the expression of receptors for guidance molecules that discern the guidance cues en route and at their putative target. Studies in our lab are aimed at elucidating axon guidance molecules that guide axons along their trajectory pathways, at junction points and at their targets (Burstyn-Cohen et al., 1999; Haimson et al., 2021b; Zisman et al., 2007). Our studies have revealed the role of transcription factors from the Lim-homeodmain family (Avraham et al., 2010a; Avraham et al., 2009; Kohl et al., 2012; Kohl et al., 2015) and receptors for axon guidance molecules (Cohen et al., 2017), in the control of axonal trajectory of genetically defined interneurons. Our future studies are aimed at elucidating the transcriptional network of Lim-HD, BarHl transcription factors in regulating the crossing of the spinal cord midline.
Avraham, O., Hadas, Y., Vald, L., Hong, S., Song, M.R., and Klar, A. (2010a). Motor and Dorsal Root Ganglion Axons Serve as Choice Points for the Ipsilateral Turning of dI3 Axons. J Neurosci 30, 15546-15557.
Avraham, O., Hadas, Y., Vald, L., Zisman, S., Schejter, A., Visel, A., and Klar, A. (2009). Transcriptional control of axonal guidance and sorting in dorsal interneurons by the Lim-HD proteins Lhx9 and Lhx1. Neural Dev 4, 21.
Cohen, O., Vald, L., Yamagata, M., Sanes, J.R., and Klar, A. (2017). Roles of DSCAM in axonal decussation and fasciculation of chick spinal interneurons. Int J Dev Biol 61, 235-244.
Burstyn-Cohen, T., Tzarfaty, V., Frumkin, A., Feinstein, Y., Stoeckli, E., and Klar, A. (1999). F-spondin is required for accurate pathfinding of commissural axons at the floor plate. Neuron 23, 233-246.
Cohen, O., Vald, L., Yamagata, M., Sanes, J.R., and Klar, A. (2017). Roles of DSCAM in axonal decussation and fasciculation of chick spinal interneurons. Int J Dev Biol 61, 235-244.
Kohl, A., Hadas, Y., Klar, A., and Sela-Donenfeld, D. (2012). Axonal patterns and targets of dA1 interneurons in the chick hindbrain J Neuroscience 32 5757-5771.
Kohl, A., Marquardt, T., Klar, A., and Sela-Donenfeld, D. (2015). Control of axon guidance and neurotransmitter phenotype of dB1 hindbrain interneurons by Lim-HD code. J Neurosci 35, 2596-2611.
Zisman, S., Marom, K., Avraham, O., Rinsky-Halivni, L., Gai, U., Kligun, G., Tzarfaty-Majar, V., Suzuki, T., and Klar, A. (2007). Proteolysis and membrane capture of F-spondin generates combinatorial guidance cues from a single molecule. J Cell Biol 178, 1237-1249.
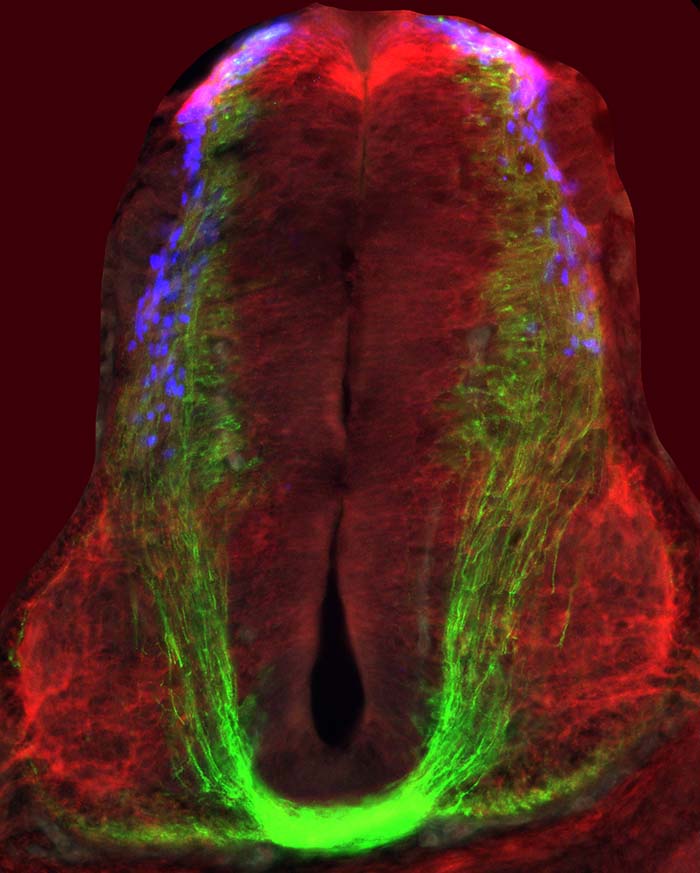
Limb alternation is the predominant mode of gait in some amphibians (salamanders), reptiles, and terrestrial mammals. In contrast, synchronous limb movements are manifested by the legs of other amphibians (frogs and toads), the wings of birds, the limbs of sea turtles, the legs of some marsupials, and the wings of bats. Flight requires synchronous flapping of wings, and in birds, it evolved from ancestral reptiles that originally used alternate stepping for locomotion. In mammals, the axonal guidance molecule ephrin-B3 is expressed in the spinal cord midline and is required for forming spinal circuits that instruct alternate stepping. Its loss leads to a hopping phenotype due to the formation of spinal network that facilitates synchronous limb movements. We found that birds lack a functional ephrin-B3 and that this loss is linked to the emergence of synchrony-mediating circuitry at spinal wing levels in birds (Haimson et al., 2021b). Our study demonstrates how naturally occurring genetic modifications affect the patterning of spinal circuitry involved in the evolution of different modes of locomotion. We plan to study the circuitry in the spinal cord of hopping, butterfly style swimming, and flying organisms; and the genome organization, expression, and function of transcription factors, guidance molecules, and their receptors. Axonal wiring is vital for the functional nervous system. However, the knowledge regarding the development of species-specific networks that evolved to support maneuvering in various environmental milieu is limited. Our present and future studies are aimed to elucidate this neural evolutionary enigma.
Haimson, B., Meir, O., Sudakevitz-Merzbach, R., Elberg, G., Friedrich, S., Lovell, P.V., Paixao, S., Klein, R., Mello, C.V., and Klar, A. (2021b). Natural loss of function of ephrin-B3 shapes spinal flight circuitry in birds. Sci Adv 7.
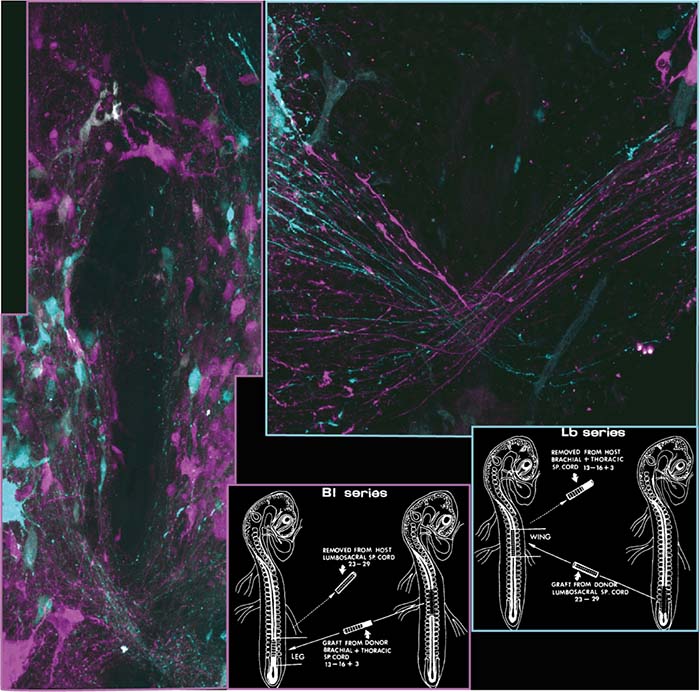
Habitual use of striding bipedal gaits in walking and running, as the primary movement mode, has evolved independently among vertebrates in primates and birds. Lack of coordination of voluntary movements, diagnosed in humans as ataxia, is attributed to degenerative changes in cerebello-spinal circuits. The cerebellum regulates the maintenance of stability, coordination, precision and timing of movements. It is postulated that internal spinal network information is relayed to the cerebellum. However, little is known about the genetic origin of these spinocerebellar tract neurons, and thus their role in stable locomotion was not addressed. Using intersectional genetics and the chicken spinal circuit-deciphering toolbox, we targeted a specific subtype of spinal INs – dI2 neurons and studied their connectome and physiological role. We found that lumbar dI2 neurons deliver peripheral and intraspinal feedback to the cerebellum. Silencing of dI2 neurons lead to destabilized stepping in P8 hatchlings, with occasional collapses, variable step profiles and a wide-base walking gait, suggesting that dI2 neurons contribute to the stabilization of the bipedal gait (Haimson et al., 2021a). Our future studies are aimed at characterizing additional genetically-defined spinocerebellar, and studying their role in bipedal and quadrupedal (mice) locomotion.
Haimson, B., Hadas, Y., Bernat, N., Kania, A., Daley, M.A., Cinnamon, Y., Lev-Tov, A., and Klar, A. (2021). Spinal lumbar dI2 interneurons contribute to stability of bipedal stepping. Elife 10.
Some of the fundamental studies in the development of the vertebrate nervous system were done using chick embryos as a model organism. These include the basic principles of neural tube patterning, neuronal cell fate determination, apoptosis, and axon guidance. The accessibility of the chick spinal cord, and the ability to surgically manipulate it, together with the evolutionary conservation of many sensorimotor circuits, make the chick embryo an ideal model system for deciphering the sensorimotor connectome. However, the lack of adequate genetic means for gene targeting in the chick embryo has hampered its use as a model organism. We have developed a unique collection of molecular tools, enabling the identification and molecular and physiological manipulation of specific neuronal circuits in the spinal cord that surmount that obstacle (Avraham et al., 2010b; Hadas et al., 2014; Hadas et al., 2013). The accessibility of the embryo and the ability to surgically and genetically manipulate the nervous system in vivo renew now the chick as a model system for studying the role of neuronal circuits in physiology and behavior. We shared the toolbox and generated new project-designated tools with other groups (Escalante et al., 2013; Lahaie et al., 2019; Mansour et al., 2014; Nitzan et al., 2013).
Avraham, O., Zisman, S., Hadas, Y., Vald, L., and Klar, A. (2010b). Deciphering axonal pathways of genetically defined groups of neurons in the chick neural tube utilizing in ovo electroporation. J Vis Exp.
Escalante, A., Murillo, B., Morenilla-Palao, C., Klar, A., and Herrera, E. (2013). Zic2-dependent axon midline avoidance controls the formation of major ipsilateral tracts in the CNS. Neuron 18, 1392-1406.
Hadas, Y., Etlin, A., Falk, H., Avraham, O., Kobiler, O., Panet, A., Lev-Tov, A., and Klar, A. (2014). A ‘tool box’ for deciphering neuronal circuits in the developing chick spinal cord. Nucleic acids research 42, e148.
Hadas, Y., Nitzan, N., Furley, A.J.W., Kozlov, S.V., and Klar, A. (2013). Distinct Cis Regulatory Elements Govern the Expression of TAG1 in Embryonic Sensory Ganglia and Spinal Cord. PLoS One 8, e57960.
Lahaie, S., Morales, D., Bagci, H., Hamoud, N., Castonguay, C.E., Kazan, J.M., Desrochers, G., Klar, A., Gingras, A.C., Pause, A., et al. (2019). The endosomal sorting adaptor HD-PTP is required for ephrin-B:EphB signalling in cellular collapse and spinal motor axon guidance. Sci Rep 9, 11945.
Mansour, A., Khazanov-Zisman, S., Y., N., Klar, A., and Ben-Arie, N. (2014). Nato3 plays an integral role in dorsoventral patterning of the spinal cord by segregating floor plate/p3 fates via Nkx2.2 suppression and Foxa2 maintenance. Development.
Nitzan, E., Krispin, S., Pfaltzgraff, E.R., Klar, A., Labosky, P.A., and Kalcheim, C. (2013). A dynamic code of dorsal neural tube genes regulates the segregation between neurogenic and melanogenic neural crest cells. Development (Cambridge, England) 140, 2269-2279.
Israel Science Foundation
BSF U.S-Israel Binational Science Foundation
Spinal lumbar dI2 interneurons contribute to stability of bipedal stepping
Baruch Haimson, Yoav Hadas, Nimrod Bernat, Artur Kania, Monica A Daley, Yuval Cinnamon, Aharon Lev-Tov, Avihu Klar
Stability, coordination, precision, and timing of movements are regulated by the cerebellum. It is postulated that internal spinal network information is relayed to the cerebellum. However, little is known about the genetic origin of these spinocerebellar tract neurons. In a collaborative study with the Lev-Tov lab, Baruch Haimson has identified a population of neurons in the chick spinal cord – dI2, that project to the cerebellum and rely a peripheral and intraspinal feedback. Silencing neuronal activity of dI2 neurons lead to destabilized stepping in post-hatching chicks. The lumbar dI2 neurons are the first genetically identifiable neurons that match the anatomical criteria of the spinocerebellar tract (VSCT) neurons and their predicted role.
Natural loss of function of ephrin-B3 shapes spinal flight circuitry in birds
Haimson B, Meir O, Sudakevitz-Merzbach R, Elberg G, Friedrich S, Lovell PV, Paixão S, Klein R, Mello CV, Klar A.
Birds have evolved from dinosaurs that use alternating gait. Their adaptation for flight includes substantial structural specializations of the forelimb and of the spinal cord circuits that enable synchronous limb movements. In a collaborative study with the Mello lab, Baruch Haimson, Reut Sudakevits-Merzbach and Gerard Elberg found that in birds the guidance molecule- ephrin-B3 is altered at multiple levels, including gene loss, modified expression patterns, and protein structure, leading to the loss of ephrin-B3 function. These mutations are linked to shifting spinal cord circuitry towards synchrony-related, which support wing flapping. This study is the 1st demonstration of the genetic changes that instructed a change is spinal circuitry that enabled the evolution of flight.
A ‘tool box’ for deciphering neuronal circuits in the developing chick spinal cord
Hadas Y, Etlin A, Falk H, Avraham O, Kobiler O, Panet A, Lev-Tov A, Klar A
Some of the fundamental studies in the development of the vertebrate nervous system were done using chick embryos as a model organism. The accessibility of the chick spinal cord, and the ability to surgically manipulate it, together with the evolutionary conservation of many sensorimotor circuits, make the chick embryo an ideal model system for deciphering the sensorimotor connectome. However, the lack of adequate genetic means for gene targeting in the chick embryo has hampered its use as a model organism. In a collaborative study with the Lev-Tov lab, Yoav Hadas developed a unique collection of molecular tools, including neuronal specific enhancers, axonal and synaptic reporters, transsynaptic viruses, neuronal activity reporters and modulators; enabling the identification and molecular and physiological manipulation of specific neuronal circuits in the nervous system. The accessibility of the embryo and the ability to surgically and genetically manipulate the nervous system in vivo renew now the chick as a model system for studying the role of neuronal circuits in physiology and behavior.
Motor and dorsal root ganglion axons serve as choice points for the ipsilateral turning of dI3 axons
Avraham O, Hadas Y, Vald L, Hong S, Song MR, Klar A
Spinal interneurons are subdivided into cardinal subtypes based on their transcriptional code. INs differ by their type of afferent input, cell body positioning along the body axes, axonal trajectory, and axonal targets. A specific axonal pathway of a neuron is governed by a transcriptional code, manifested by the expression of receptors for guidance molecules that discern the guidance cues en route and at their putative target. To study the role of transcription factors (TFs) in the wiring of spinal INs, Oshri Avraham initially decoded the projection pattern of three spinal INs, dI1, dI2, and dI3, by utilizing novel experimental paradigms for expression in specific-INs. Subsequently, we demonstrated that IN-specific TFs of the LIM-HD family instruct the wiring of dI1-3 INs.
Control of axon guidance and neurotransmitter phenotype of dB1 hindbrain interneurons by Lim-HD code
Kohl A, Marquardt T, Klar A, Sela-Donenfeld D
Similar to the spinal cord, interneurons in the hindbrain are also subdivided into cardinal subtypes, similar to the spinal subpopulations. The hindbrain interneurons give rise to nuclei within the brainstem that process and mediate facial sensorimotor information. Together with Prof. Dalit Sela-Donenfeld (The Koret School of Veterinary Medicine) and a joint Ph.D. student – Ayelet Kohl, we decoded the wiring of two hindbrain populations (dA1 and dB1) and demonstrated the requirement of LIM-HD TFs in establishing their circuitry.

Research associate and lab manager
The function and mechanism of actions of guidance molecules determining neuronal circuits in the spinal cord.

MD-PhD student
Transcriptional and molecular control of neuronal midline crossing in the spinal cord.

M.Sc. student

undergraduate student for Biomedical sciences
a. Bachelor’s degree courses
#94634 The Fundamentals of Embryonic Development [Biomedical Sciences]. Coordinator
#94662 Comparative Developmental Biology [lab course in Biomedical Sciences]. Coordinator
#91309 Embryology and Teratology [Nursing]. Coordinator
#96212 Medical Embryology and Teratology [Medicine and Dentistry].
#94645 Neuronal Mechanisms Underlying Complex Brain Behaviors [Biomedical Sciences].
#94668 Biochemistry-Gene Structure and Expression [Biomedical Sciences].
#94113 An Overview of the Research in the Brain Research Track [Biomedical Sciences].
#94651 B.Sc. Seminars – Brain research [Biomedical Sciences].
b. Master’s degree courses
#98122 Development of the Nervous System [Biomedical Sciences]. Coordinator

For more information, please contact Avihu Klar at avihu.klar@mail.huji.ac.il
Website designed by toornet
Born 1957, Tel Aviv
Ph.D. 1989, Hebrew University
Lect. – 1993
Senior Lect – 2002
Associate Prof. – 2011
Professor – 2022