
Professor
Identification of endogenous cardiac steroids in mammalian tissue; The biological consequences of the interaction of cardiac steroids with the sodium-potassium-ATPase; Biosynthesis of the cardiac steroids in the adrenal gland; Effects of endogenous sodium-potassium-ATPase inhibitors on cell differentiation; Determination of the levels of endogenous sodium-potassium-ATPase inhibitors in pathological states, including hypertension, preeclampsia; malignancies (cancer) and manic depressive illnesses; Involvement of the sodium-potassium–ATPase/cardiac steroids system in depressive disorders; Involvement of the sodium-potassium-ATPase/cardiac steroids system in cardiac function; Involvement of intestinal signals in the regulation of phosphate homeostasis; Volume regulation and its involvement in the mitogenic response.
Identification of endogenous cardiac steroids in mammalian tissue; The biological consequences of the interaction of cardiac steroids with the sodium-potassium-ATPase; Biosynthesis of the cardiac steroids in the adrenal gland; Effects of endogenous sodium-potassium-ATPase inhibitors on cell differentiation; Determination of the levels of endogenous sodium-potassium-ATPase inhibitors in pathological states, including hypertension, preeclampsia; malignancies (cancer) and manic depressive illnesses; Involvement of the sodium-potassium–ATPase/cardiac steroids system in depressive disorders; Involvement of the sodium-potassium-ATPase/cardiac steroids system in cardiac function; Involvement of intestinal signals in the regulation of phosphate homeostasis; Volume regulation and its involvement in the mitogenic response.
Cardiac Steroids and the Na+, K+-ATPase
Cardiac steroids, such as ouabain, digoxin and bufalin are hormones synthesized by and released from the adrenal gland and the hypothalamus. These compounds, the structure of which resembles that of plant and amphibian and butterfly steroids, interact only with the plasma membrane Na+, K+-ATPase. This interaction elicits numerous specific biological responses affecting the function of cells and organs.
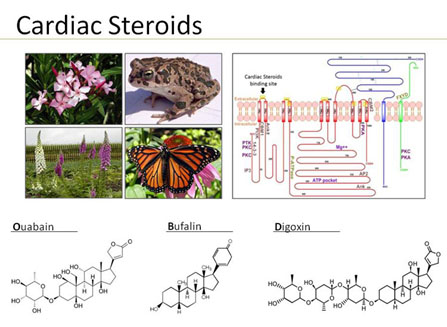
Depressive disorders, including major depression, dysthymia and bipolar disorder, are a serious and devastating group of diseases that have a major impact on the patients’ quality of life, and pose a significant concern for public health. The etiology of depressive disorders remains unclear. The Monoaminergic Hypothesis, suggesting that alterations in monoamine metabolism in the brain are responsible for the etiology of depressive disorders, is now recognized as insufficient to explain by itself the complex etiology of these diseases. Data from our and other laboratories has provided initial evidence that endogenous cardiac steroids and their only established receptor, the Na+, K+-ATPase, are involved in the mechanism underlining depressive disorders, and BD in particular.
Our recent studies in this project (Eur. Neuropsychopharmacol. 22:72-729, 2012; Front Endocrinol (Lausanne). 5:20, 1-13, 2014; Bipolar Disorders. 18:451-459, 2016; Brain Res. Bull. 137:356-362, 2018) showed that drugs affecting the Na+, K+-ATPase/cardiac steroids system are beneficial for the treatment of depression and that protection from oxidative stress is involved in this phenomenon. Hence our work is in accordance to the proposition that mal functioning of the Na+, K+-ATPase/cardiac steroids system may be involved in manifestation of depressive disorders and identify new compounds as potential drug for the treatment of these maladies.

The classical and best documented effect of cardiac steroids, as their name implies, is to increase the force of contraction of heart muscle. Indeed, cardiac steroids were widely used in Western and Eastern clinical practices for the treatment of heart failure and atrial fibrillation. Despite extensive research, the mechanism underlying cardiac steroids actions have not been fully elucidated. The dogmatic explanation for cardiac steroids-induced increase in heart contractility is that the inhibition of Na+, K+-ATPase by the steroids causes an increase in intracellular Na+ which, in turn, attenuates the Na+/Ca++ exchange, resulting in an increased intracellular Ca++ concentration, and hence greater contractility. However, recent observations led to the hypothesis that the ability of cardiac steroids to modulate a number of intracellular signaling processes, including that of extracellular-signal-regulated kinase (ERK) and PI3K-Protein Kinase B (AKT) pathways may be responsible for both short- and long-term changes in CS action on cardiac function. In recent studies on zebrafish (J Pharmacol Exp. Ther. 357:345-56, 2016) and rat (J. Cardiovasc. Pharmacol. Ther. 24: 78-89, 2019) experimental models, we proved this concept by demonstrating that the addition of ERK inhibitors (in-vitro and in-vivo) attenuated the CS-increase in heart contractility. Surprisingly, we have discovered that the combined treatment with CS and AKT inhibitors has beneficial effect as compared to the treatments with CS alone (J. Cardiovasc. Pharmacol. Ther. 24: 78-89, 2019). Treatment of hearts, in-vivo (or cardiomyocytes and isolated heart, in-vitro) resulted in a positive inotropic effect of the CS at doses that did not have any effects when administered alone (sub-nanomolar). This beneficial effect of the combined treatment with CS and AKT inhibitors was now proven in several experimental systems including, primary cardiomyocytes from zebrafish heart, zebrafish heart contractility in-vivo, primary cardiomyocytes from rat heart, isolated rat heart contractility (Langendorff preparation) and experiments on heart contractility following LAD ligation in rats. Hence, taken together, these studies establish a strong proof-of-principal for the development of the combined treatment for clinical use for the treatment of heart failure.
Horesh, N., Pelov, I., Pogodin, I., Zannadeh, H., Rosen, H., Mikhrina, A. L., … & Lichtstein, D. (2024). Involvement of the Na+, K+-ATPase α1 Isoform and Endogenous Cardiac Steroids in Depression-and Manic-like Behaviors. International Journal of Molecular Sciences, 25(3), 1644.
DOI: https://doi.org/10.3390%2Fijms25031644
Singh SV, Fedorova OV, Wei W, Rosen H, Horesh N, Ilani A, Lichtstein D. Na+, K+-ATPase α Isoforms and Endogenous Cardiac Steroids in Prefrontal Cortex of Bipolar Patients and Controls. Int J Mol Sci. 21(16):E5912 (2020)
Here, we address the hypothesis that the α isoforms of the Na+, K+-ATPase and its regulators are altered in the prefrontal cortex of bipolar disease patients. We found that the α2 and α3 isoforms were significantly higher and marinobufagenin levels were significantly lower in the prefrontal cortex of the bipolar disease patients compared with those in the control. A positive correlation was found between the levels of the three α isoforms in all samples and between the α1 isoform and ouabain levels in the controls. These results are in accordance with the notion that the Na+, K+-ATPase-endogenous cardiac steroids system is involved in bipolar disease and suggest that it may be used as a target for drug development.
Buzaglo N, Golomb M, Rosen H, Beeri R, Ami HC, Langane F, Pierre S, Lichtstein D. Augmentation of Ouabain-Induced Increase in Heart Muscle Contractility by Akt Inhibitor MK-2206. J. Cardiovasc. Pharmacol. Ther. 24: 78-89 (2019).
Here we test the hypothesis that combined treatment with ouabain and Akt inhibitor (MK-2206) augments ouabain-induced inotropy in mammalian models. We demonstrate that the combined treatment led to an ouabain-induced increase in contractility at concentrations at which ouabain alone was ineffective. Furthermore, cell viability experiments revealed that this treatment protected primary cardiomyocytes from MK-2206 toxicity and in vivo reduced the size of scar tissue 10 days post-LAD ligation. We propose that Akt activity imposes a constant inhibitory force on muscle contraction, which is attenuated by low concentrations of MK-2206, resulting in potentiation of the ouabain effect. This demonstration of the increase in the CS effect advocates the development of the combined treatment in CHF.
Lichtstein D, Ilani A, Rosen H, Horesh N, Singh SV, Buzaglo N and Hodes A. Na⁺, K⁺-ATPase Signaling and Bipolar Disorder. Int. J. Mol. Sci. 219:1-13 (2018).
Here, evidence for the participation of Na+, K+-ATPase and its endogenous regulators, the endogenous cardiac steroids (ECS), in the etiology of Bipolar disorder is reviewed. Proof for the involvement of brain Na+, K+-ATPase and ECS in behavior is summarized and it is hypothesized that ECS-Na+, K+-ATPase-induced activation of intracellular signaling participates in the mechanisms underlying BD. We propose that the activation of ERK, AKT, and NFκB, resulting from ECS-Na+, K+-ATPase interaction, modifies neuronal activity and neurotransmission which, in turn, participate in the regulation of behavior and BD. These observations suggest Na+, K+-ATPase-mediated signaling is a potential target for drug development for the treatment of BD.
Hodes A, Lifschytz T, Rosen H, Cohen Ben-Ami H, Lichtstein D. Reduction in endogenous cardiac steroids protects the brain from oxidative stress in a mouse model of mania induced by amphetamine. Brain Res. Bull. 137:356-362 (2018).
The aim of the present study was to examine the role of brain oxidative stress in the CS-induced behavioral effects in mice. AMPH administration resulted in a marked hyperactivity and increased oxidative stress, as manifested by increased SOD activity, decreased activities of CAT and GPx, reduced levels of NPSH and increased levels of TBARS and PC. The administration of anti-ouabain antibodies, which reduced the AMPH-induced hyperactivity,protected against the concomitant oxidative stress in the brain. Our results demonstrate that oxidative stress participates in the effects of endogenous CS on manic-like behavior induced by AMPH. These findings support the notion that CS and oxidative stress may be associated with the pathophysiology of mania and BD.
Dvela-Levitt M, Cohen-Ben Ami H, Rosen H, Ornoy A, Hochner-Celnikier D, Granat M, and Lichtstein D. Reduction in maternal circulating ouabain impairs offspring growth and kidney development. J. Am. Soc. Nephrol. 26:1103-14 (2015).
We show that intraperitoneal administration of anti-ouabain antibodies to pregnant mice resulted in a .80% decline in the circulating ouabain level. This reduction caused a significant decrease in offspring body weight, accompanied by enlargement of the offspring heart and inhibition of kidney and liver growth. Kidney growth inhibition was manifested by a decrease in the size and number of nephrons. After the reduction in maternal circulating ouabain, kidney expression of cyclin D1 was reduced and the expression of the a1 isoform of the Na+, K+-ATPase was increased. In addition, the elevation of proliferation signals including ERK1/2, p-90RSK, Akt, PCNA, and Ki-67, and a reduction in apoptotic factors such as Bax, caspase-3, and TUNEL were detected. During human pregnancy, the circulating maternal ouabain level increased and the highest concentration of the steroid was found in the placenta. Furthermore, circulating ouabain levels in women with small-for-gestational age neonates were significantly lower than the levels in women with normal-for-gestational age newborns. These results support the notion that ouabain is a growth factor and suggest that a reduction in the concentration of this hormone during pregnancy may increase the risk of impaired growth and kidney development.
Dvela-Levitt M, Ami HC, Rosen H, Shohami E, Lichtstein D. Ouabain improves functional recovery following traumatic brain injury. J. Neurotrauma. 31(23):1942-7. (2014).
We studied the effects of ouabain on mouse recovery following closed head injury (CHI), a model for traumatic brain injury. We show that chronic, but not acute, intraperitoneal administration of a low dose of ouabain significantly improves mouse recovery and functional outcome. The improvement in mouse performance was accompanied by a decrease in lesion size. In addition, mice that underwent CHI and were treated with ouabain showed an increase in the number of proliferating cells in the subventricular zone and in the area surrounding the site of injury. Determination of the identity of the proliferating cells in the area surrounding the trauma showed that whereas there was no change in the proliferation of endothelial cells or astrocytes, neuronal cell proliferation almost doubled in the ouabain-treated mice in comparison with that of the vehicle animals. These results point to a neuroprotective effects of low doses of ouabain and imply its involvement in brain recovery and neuronal regeneration. This suggests that ouabain and maybe other cardiac steroids may be used for the treatment of traumatic brain injury.

Post-Doctoral Fellow
Investigation of metals roles in bipolar disorder and their involvement in Na+ K+ ATPase activity
Noa Rosenthal-Horesh, PhD.
Website designed by toornet
Education
1970 B.Sc. in Physiology and Zoology, The Hebrew University, Jerusalem, Israel
1970-1972 M.Sc. in Physiology, Department of Physiology, The Hebrew University, Hadassah Medical School, Jerusalem, Israel.
1973-1977 Ph.D., Department of Physiology, Hebrew University Hadassah Medical School, Jerusalem, Israel. (Thesis: “Increased Production of Gamma Aminobutyryl choline in Cerebral Cortex Caused by Afferent Electrical Stimulation” (Thesis Advisors: Prof. J. Dobkin and Prof. J. Magnes).
1977-1979 Postdoctoral Fellow, Department of Physiological Chemistry and Pharmacology, Roche Institute of Molecular Biology, Nutley, New Jersey, U.S.A.
Positions held
1970-1972 Teaching and Research Assistant, Department of Physiology, The Hebrew University, Hadassah Medical School, Jerusalem, Israel
1972-1974 Assistant Instructor, Department of Physiology, The Hebrew University, Hadassah Medical School, Jerusalem, Israel
1975-1977 Instructor, Department of Physiology, The Hebrew University, Hadassah Medical School, Jerusalem, Israel
1977-1979 Postdoctoral Fellow, Department of Physiological Chemistry and Pharmacology, Roche Institute of Molecular Biology, Nutley, New Jersey, U.S.A.
1979-1983 Lecturer, (REVSON fellowship) Department of Physiology, The Hebrew University, Hadassah Medical School, Jerusalem, Israel
1981 (summer) Visiting Scientist, Department of Physiological Chemistry and Pharmacology, Roche Institute of Molecular Biology, Nutley, New Jersey, USA
1983-1987 Senior Lecturer, Department of Physiology, The Hebrew University Hadassah Medical School, Jerusalem, Israel.
1985-1986 Visiting Scientist, Laboratory of Theoretical and Physical Biology, NICHD, National Institutes of Health, Bethesda, Maryland, USA
1988-1994 Associate Professor, Department of Physiology, The Hebrew University Hadassah Medical School, Jerusalem, Israel
1994-present Professor of Physiology, Department of Physiology, The Hebrew University Hadassah Medical School, Jerusalem, Israel
1997-1998 Visiting Scientist, Laboratory of Mechanisms of Ocular Diseases, NEI, National Institutes of Health, Bethesda, Maryland, USA
2007 (summer) Visiting Professor, Department of Physiology, Pharmacology, Metabolism and cardiovascular Sciences, Medical Center University of Toledo, Toledo, Ohio, USA
2007-2011 Jacob Gitlin Chair in Physiology, The Hebrew University, Jerusalem, Israel
2011-present Walter & Greta Stiel Chair in Heart Studies, The Hebrew University, Jerusalem
Professional Membership
1979-present International Society of Neurochemistry
1979-present Israel Society for Physiological and Pharmacological
1980-present Society of Neurosciences (Europe)
1986-present The American Society of Hypertension
1992-present Israeli Society for Neurosciences
1999-present The American Physiological Society
Editorial Tasks
University and Other Activities
1982-1985 Chairman of the Neurobiology Teaching Division, The Hebrew University, Jerusalem
1988-1994 Elected representative of the Senior Lecturers and Associate Professors for the University Senate
1989-1997 Member of the admission committee of the Medical School, The Hebrew University, Jerusalem
1990-1996 Member of the Committee for cellular biology of the graduate studies, The Hebrew University, Jerusalem
1992-1996 Member of the Teaching Committee, Faculty of Medicine, The Hebrew University, Jerusalem
1992-1996 Chairman, Department of Physiology, The Hebrew University, Hadassah Medical School, Jerusalem
1994-1997 Member of the Committee for graduate studies, The Hebrew University, Jerusalem
1992-2002 Member of the Management Committee of The Institute for Medical Sciences, Faculty of Medicine, The Hebrew University, Jerusalem
1996-1999 President of the Israel Society for Physiology and Pharmacology
1998-2002 Chairman, Institute of Medical Sciences, The Hebrew University, Hadassah Medical School, Jerusalem
1999-2002 Member of the Planning and Development Committee of the Faculty of Medicine, The Hebrew University, Jerusalem
2007–2013 Elected member of the Senate to the Executive Committee of the Hebrew University
2008-2012 Member of the Planning and Development Committee of the Faculty of Medicine, The Hebrew University, Jerusalem
2008-2012 Chairman, Institute for Medical Research Israel-Canada, The Hebrew University, Hadassah Medical School, Jerusalem
2009–2013 Elected member of the Senate to the Executive Committee of the Hebrew University
2013-2017 Dean Faculty of Medicine, The Hebrew University of Jerusalem