
Associate Professor
The common theme to our research is the vomeronasal system (abbreviated: VNS), a chemosensory system that is present in most vertebrates, and which specializes in processing signals from other organisms. These signals include pheromones (stereotyped cues from other individuals of the same species), but are not combined to them. Above all, we want to understand how the VNS makes sense of the very complex signals that activate it. We are using electrophysiological and behavioral approaches. Using electrophysiology, we measure neuronal responses in the VNS (and primarily in the accessory olfactory bulb, AOB, the first brain stage of the system) after presentation of various stimuli that are relevant for behavior. We aim to understand how information about the donors of these stimuli are represented by neuronal activity. Using behavioral approaches and optogenetics, we are also interested in the capacity of the VNS to form new stimulus response associations. Ultimately, we would like to understand the neuronal mechanisms that underlie the ability to form new stimulus response associations.
In a nutshell, we use electrophysiology, behavioral analysis, (and sure, optogenetics…) to test how information is processed by the mouse vomeronasal system. Below is a detailed description of our research interests, which roughly correspond to projects and publications. We begin with the basics and then cover more specific topics.
For many systems neuroscientists, the most exciting questions about the brain concern its function as a computing device: a device that receives sensory information and produces a behavioral, physiological, or internal (mental) result (a change of internal state, if you will). Numerous brain functions fall into this description, and we study one of them: the processing of chemical cues from other organisms, to guide behavioral or physiological outputs. For many animals, this is a fundamental ability that is absolutely crucial for survival, as it guides responses to other organisms, whether conspecifics including mates, competitors or offspring, potential predators, or prey.
The general problem we are interested in is described in a figure from this hypothesis article.
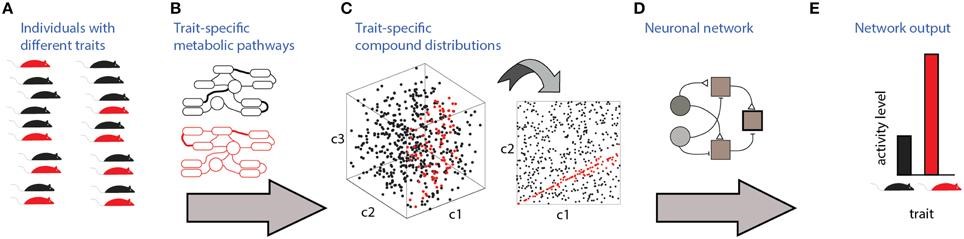
An outline of our research. See this for more details.
The idea in this scheme is that organisms must derive information about other organisms to guide proper (or improper, we don’t judge…) behavioral or physiological outputs. This requires that information about other organisms’ traits (i.e. their species, sex, physiological state, individuality, and so on) will be reflected by the molecules that they emit. The brain’s task then is to detect these chemical profiles, identify those that are informative for a specific aspect, and then use that information to act accordingly. In simple terms, much of our research involves recording neuronal activity (measuring stage “E” above) while presenting stimuli (collected from organisms as in “A”) to our subject animals, and then try to figure out the complex processes that are represented by the single arrow in “D”.
Vertebrates use more than a single system for chemosensation, and our focus is on the vomeronasal system (VNS), also known as the accessory olfactory system (AOS). See this review for a description of the VNS. The VNS is present in most vertebrates and is prominent in rodents. One motivation for focusing on it is that we are quite confident about its role: to process cues from other organisms. This is not to be taken for granted as we cannot always assign a well-defined function to each of the known brain regions/structures.
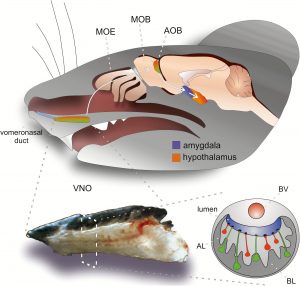
Some key structure of the VNS. Taken from a relatively recent review.
Numerous studies (not ours) have shown that deficits in the VNS can lead to significant impairments associated with interactions with other organisms, whether conspecifics (from the same species) or heterospecifics (from other species). The VNS is compact, both in size, and in the number of processing stages between sensory stimulus and hypothalamic neurons that control specific outputs. The compactness facilitates a thorough analysis of the system, but there are also inherent challenges in studying it. One of these involves the system’s mode of sampling. Briefly, unlike the main olfactory system which continuously samples stimuli via breathing, VNs stimulus uptake is an active process that requires suction of solubilized stimuli into the sensory organ of the VNS, the vomeronasal organ (VNO) in the nasal cavity. In some animals, such as horses (but not mice unfortunately), VNO stimulus uptake is externally visible.

We are not studying horses, but horses also have a VNO and when they activate it, it can be seen from the outside. Photo credit to Brandinian.
Our approach to induce stimulus update to the VNS involves electrical stimulation of the sympathetic nerve trunk. A few other labs in the world developed related preparations, each with their own pros and cons. Our approach involves a surgical procedure during which we implant a cuff electrode around the sympathetic nerve trunk, allowing us to repeatedly present stimuli to the VNS and measure responses to it. The preparation was described here in a lot of detail.
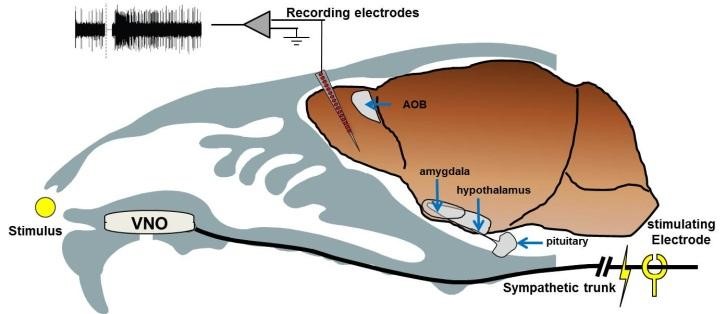
A schematic illustration of our preparation
Example of neuronal responses to social stimuli are shown below:
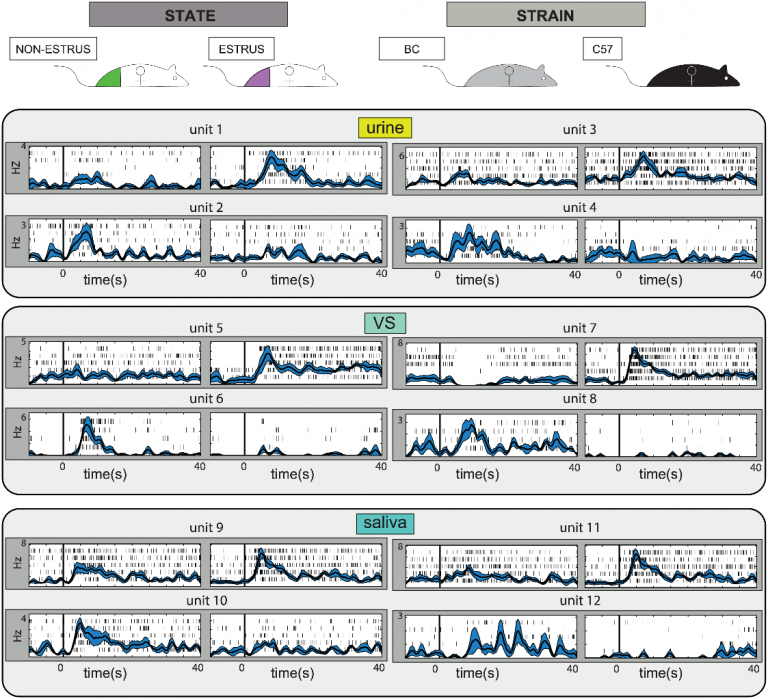
The image, taken from a study by Dr. Anat Kahan, shows responses from several AOB units, each of which is responsive to one of the two presented stimuli. Neurons on the left seem selective to the stimulus donor’s physiological state (in estrus, or not in estrus) whereas those on the right seem selective to the stimulus donor’s strain (BC, for Balb/C or C57 for C57Black/6). One notable feature of AOB responses is their very long duration. We have recently studied the temporal dynamics of AOB responses, and concluded that considering fine temporal dynamics does not significantly aid decoding of stimulus information. Regardless of temporal dynamics, note that for each trait (estrus, non-estrus, BalbC, or C57) we find at least one neuron that increases its firing rate. Each frame shows responses to one stimulus source: urine, vaginal secretions (VS), and saliva. The figure highlights the fact that AOB neurons are indeed sensitive to the donors’ identity and physiology, and that this information can be derived from multiple secretions.
Looking at these figures one might conclude that detecting relevant stimulus features is simple: one only has to probe one of these neurons shown on the left to determine an animals’ physiological state, or at one of the neurons on the right to detect its strain. But, this is a false conclusion. To illustrate, consider neuron 1 on the upper left side: this neuron responded to a urine sample collected from estrus females and did not respond to another urine sample from non-estrus females. Yet, these are specific samples, from certain mice, at a certain age, at a certain reproductive status, health status, and so on and on. Also, these are samples of one bodily secretion, urine. There is no basis to conclude that this neuron will respond selectively to any sample (urine, vaginal secretion, saliva, tear glands, etc…) from any estrus female, and not respond to any sample from a non-estrus female. In other words, based on this data, one should not confound selectivity to one feature in a limited stimulus set (and all sets are limited…) with general selectivity to the feature. In attempt to analyze this issue systematically we tested the ability of classifiers to extract behaviorally relevant information from AOB neurons. Our conclusion is illustrated below (referring to the old tale of the blind men and the elephant):

We are also not working on elephants either, but we think that this image captures how the AOB can represent information about other organisms. The image (by Yaron Ben-Shaul) accompanied this paper.
In words, we concluded that retrieving information about behaviorally relevant features requires pooling information from multiple neurons. Why is this important? Because it is a departure from how people sometimes think of pheromone based communication. Pheromones are often equated with social signals, but this is not justified. In fact, pheromones represent just one class of social signals, and are appropriate for eliciting stereotypic responses to stereotypic stimuli. This involves not only the nature of the pheromone molecule, but also the neuronal circuits required to detect and respond to it. For example, a pheromone is a suitable cue for eliciting an unconditional mating response between a male and a female, but not for conveying subtler information such as individuality, or specific physiological states.
These observations set the context for our research and analyses and building upon this understanding of the system, we are interested in several other questions related to the function of the VNS.
Although we think that information is distributed across multiple neurons, we are still interested in understanding which stimulus features are encoded by the activity of AOB neurons, and ultimately, how these are related to the chemical composition of the stimuli. In this context, we note that there is something surprising about the wiring of the vomeronasal system. I for one, would expect an orderly organization in a system that is supposed to detect well-defined cues. But, looking at the AOB, one gets the impression that the wiring is largely random: consider this image of the olfactory bulb, made by Santiago Ramon y Cajal:
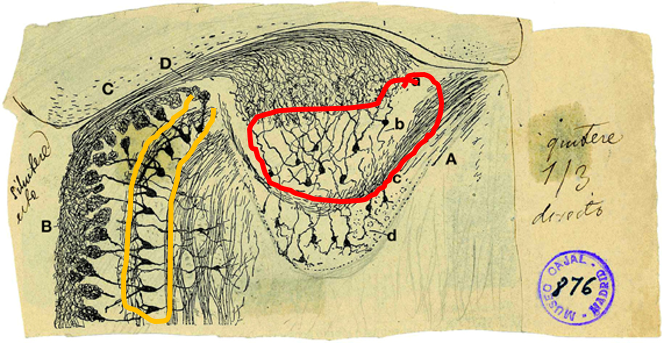
Ramon y Cajal, S. (1901). Textura del lóbulo olfativo accesorio. Trab. Lab. Invest. Biol. 1, 141–149.
The orange boundary encloses cell bodies of mitral tufted cells of the main olfactory bulb. The red boundary shows cell bodies of mitral tufted cells of the accessory olfactory bulb. While both neuron classes are the output neurons, there are clear differences. Main olfactory bulb mitral cells are larger, more uniform in their position, and perhaps most importantly, they send dendrites to sample information from a single glomerulus. In contrast, accessory olfactory bulb mitral tufted cells send their dendrites to multiple glomeruli. Closer inspection shows that individual neurons can sample inputs from multiple glomeruli (between 1 and 10). Note that in both systems, each glomerulus contains axons from receptor neurons that express a single type of receptor (as always in Biology, there may be some exceptions to this “rule”, but it is a good approximation in both systems). In the main olfactory system, the receptor neurons reside in the nasal cavity, in the VNS, they reside in the vomeronasal organ. So, this implies that whereas MOB mitral cells roughly inherit the tuning properties of single glomeruli (the glomeruli they sample input from), receptive fields of AOB mitral tufted cells may be considerably more complex. It is very hard to deduce receptive properties from connectivity patterns, but the images shown in the figure above, and others like it, suggest that AOB mitral tufted cells may have complex receptive profiles. But what are these response profiles? One possibility is that AOB neurons sample specific combinations of molecules that when present together unequivocally report some feature. Another is that AOB neurons sample glomeruli entirely randomly. While some evidence suggests that sampling is not entirely random, the overall picture is not clear. Ideally, one could record from individual neurons while presenting numerous combinations of molecules and measuring the neuronal responses. If for each AOB mitral tufted cell, we knew how it responded to any combination of molecules, and what each stimulus combination implies about the organism that emitted it, then we would have a very good understanding of the information that the AOB sends to its downstream targets. But this is not feasible… and thus we have to make do with simpler, and more indirect ways to learn about the receptive fields of these neurons. Given our current experimental abilities, the most productive approach is to carefully select stimulus sets and then to examine which stimulus features are best represented by neuronal activity. Using a new analyses approach that we developed, we are currently analyzing data from several lines of experiments to reveal which stimulus features are best represented by this data.
The image of the messy AOB circuitry raises another major question, which is especially significant for a system designed to deal with signals that have an inherent meaning (as opposed to a system designed to form flexible associations between initially neutral cues and behavioral outputs). Presumably, signals with innate meanings should be mapped in a manner that is stereotypic across individuals. This is related to a “deep”, maybe even philosophical question concerning subjective experiences. As humans, we can agree on what is red and what is green, or what is an orange smell and what is an apple smell. We can do this because we each have a representation of red and green and orange smell and apple smell, and when we say that something say is red it merely means that it is similar (we are not going to define what similar is) to our internal representation of red. But, there is no way to know, and perhaps no sense in asking, whether any two individuals’ red are the same red, because this is a subjective perception. So, we can agree on distances in perceptual space but not on “absolutes”, and maybe distances are all that matter, or at least required. On a neuronal level, one actually could test if a given stimulus elicits similar responses in different individuals, and work from the labs of Larry Katz and Markus Meister has shown that in the case of the mouse and rat main olfactory bulb, this is largely the case. In the AOB it is much harder to make a comparison between absolute response patterns, both because it is less organized and because it’s hidden location makes it hard to analyze the spatial location of responding neurons. Thus, we compare relative distances (in neuronal, not spatial space) between population level representations. Instead of doing it across individuals, we compared strains of mice (which can be considered a model for different individuals that differ in their genetic makeup and receptor repertoire). The figure below, from a recent paper led by Dr. Rohini Bansal, part of collaborative project with the labs of Marc Spehr, Pavel Stopka and Tali Kimhi, shows that indeed, population level representations are similar across individuals from the two strains, leading us to the conclude that indeed all mice smell the same, that is, they smell the world in a similar manner. Eventually, we would like to test whether this similarity holds for more complex stimuli with subtler distinctions between them (i.e. animals that differ by physiological state rather than sex).
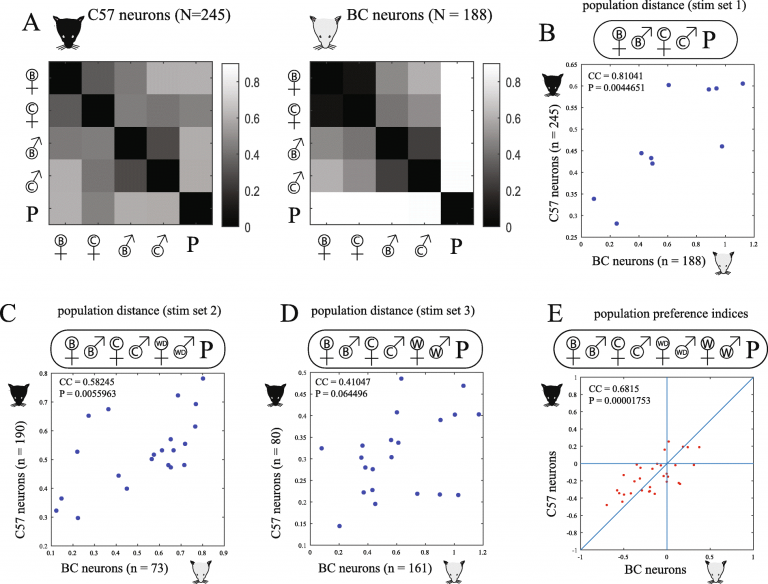
The title of the paper (“Do all mice smell the same? Chemosensory cues from inbred and wild mouse strains elicit stereotypic sensory representations in the accessory olfactory bulb”) is intentionally ambiguous. The ambiguity refers to the second theme of the paper, which is entirely distinct from the first and which addresses a more evolutionary flavor. Specifically, we asked whether mice emit the same smell. More accurately, we asked whether stimuli from inbred and wild mice elicit similar responses at the level of the AOB. Our answer to this question is also positive. Thus, despite the evolutionary distinct pathways of lab and inbred mice, the responses elicited by their urine stimuli are overall similar, so that a male can be easily identified as a male, and a female as a female. This is not to say that there are no differences between wild and inbred mouse secretions. There are differences, and these differences are more prominent for wild caught mice, as opposed to wild derived mice, bred several generations in captivity. But, responses to urine stimuli from for both types of mice, cluster with inbred stimuli of the same sex (see the figure below, another image from the same manuscript).
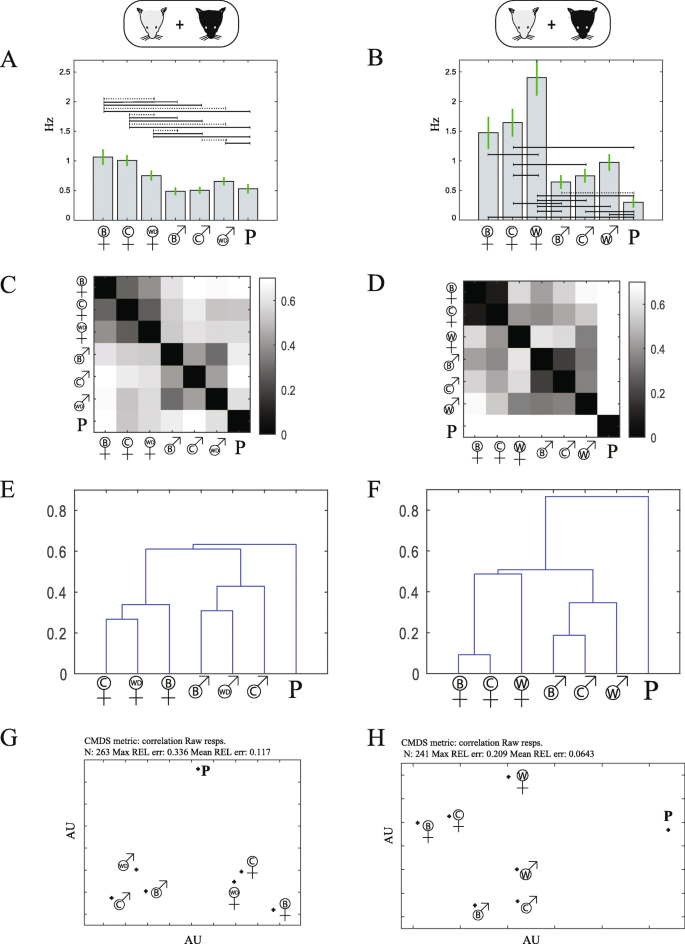
Association of the VNS with innately meaningful cues might imply that experience and plasticity play only a minor role in its function. However, this is not the case. Responses to innately relevant stimuli could well depend on organisms’ current state and previous experience. Furthermore, associative learning can endow a strong meaning to an initially neutral cue, resulting in a robust response to that cue. More generally, while all organisms engage in similar innate behaviors (eating, reproducing, competing for resources, evading danger…) the relevant stimuli that motivate or elicit such responses differ among species and among individuals within a species. In fact, the most fascinating aspect of innate behavior is perhaps the interplay between the common (almost everybody likes to eat) and the individual (different individuals enjoy different foods at different circumstances). Below is a description of some of the specific projects that we are currently pursuing under the general theme of plasticity.
Associative learning in an “innate” system
On the spectrum spanned by entirely innate to completely learned, one extreme is a scenario in which a given stimulus elicits a hardwired rigid response. While most people would not claim that the VNS is entirely a hardwired system, it was not known how readily arbitrary activation patterns can be associated with an initially neutral response. Recently, in work led by Dr. Karen Marom, we tested the ability of mice to learn to associate VNS activity with a learned response. The major challenge was technical: activating the VNS without activation of the MOS. Activation by natural stimuli is not a good option since any chemical stimulus might affect sensory neurons in each of the two systems. Instead, we adopted an optogenetic approach in which we bypassed the chemical stimulus and directly activated sensory neurons, or mitral cells of the VNS. Our conclusion is straightforward: the VNS can learn novel stimulus response pairing as easily as the main olfactory system does.
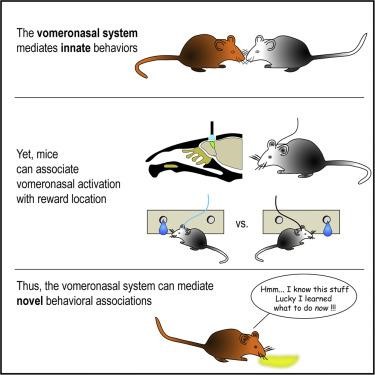
The VNS can learn novel stimulus response pairings. Graphical abstract for our paper
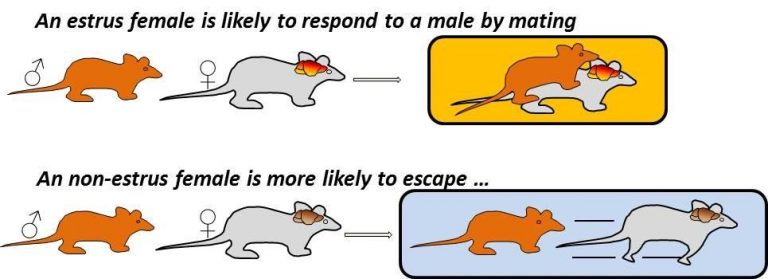
A schematic of state dependent sexual responses in female mice
Another project, which also falls under the category of state dependent processing involves the Bruce effect (also known as the pregnancy block effect).
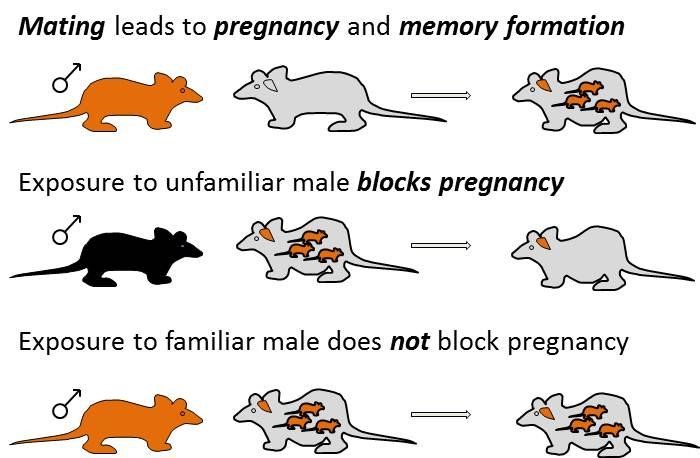
A schematic of the Bruce Effect
When a pregnant female mouse is exposed to a strange male, there is a high chance that the pregnancy will be aborted. Pregnancy block is supposedly due to a cascade of neuronal events that involves the AOB, amygdala and the hypothalamus, and leads to a decrease in level of prolactin, a hormone that is required for maintenance of pregnancy during the initial 3 days or so. But… the really interesting question is why does exposure to the father, the stud male, not cause block. One possible solution to this problem is to simply quench responses to the stud male. In fact, this is a leading theory, known as the negative template hypothesis. The idea is that after mating, that male will no longer activate neurons in the AOB, and thus would not trigger that cascade of events. But there is a problem with this theory (actually more than one). First, does that mean that the female will not be able to smell the future father of her children, at least via the VNS? That seems unlikely… But, a more fundamental problem is that silencing responses to the stud male will inevitably effect neuronal responses (i.e. representations) of other stimuli. This happens due to the combinatorial nature of responses that was described above (the elephant), which means that most neurons will respond not to one, but to multiple stimuli, and sometimes to multiple stimuli with very distinct implications. This implies that changing representations of one stimulus will also change the representation of other stimuli, and this is clearly a problem as it implies that once a female has mated, representations of many stimuli in the AOB will be modified. This will require downstream processing stages, those that actually read information from the AOB, to revise how they decode AOB signals. Again, this does not seem like a very efficient solution. In a recent manuscript by Michal Yoles-Frenkel (a collaboration with Ian Davison at BU and Steve Shea at CSHL) we suggest a way out of this problem (https://www.biorxiv.org/content/10.1101/2021.10.27.466073v1). Analyzing recordings from mated and unmated female mice, we show that if we consider activity following a single presentation of each stimulus (which is nevertheless quite long in itself, see below), then neuronal representations are very stable. In other words, there are no significant changes associated with mating, not to the stud male, nor to other stimuli. However, repeated presentation of the stud stimulus does lead to diminished responses, and this happens selectively in response to the stud male. This implies a separation into two time scales: one which is relevant for ongoing investigations, much as we engage in as we encounter many individuals in our day to day, and another which is relevant for a closer, more prolonged interaction. It is the second of these which corresponds to the interaction required for inducing pregnancy block. This solution, which is consistent with earlier in vitro work from the Davison lab, can account for the protection from the pregnancy blocking effect of the stud, while at the same time preserving the ability of the VNS to faithfully represent stimuli as required for ongoing behavioral decisions.
Temporal features of AOB neuronal responses
Temporal coding (which can mean different things to different people), is a popular theme in neuroscience. A distinction is made between temporal and rate coding, and while almost everyone agrees that this is not a clear dichotomy, it is valid to ask about the relevant time scales that convey information about the stimulus. In the main olfactory system, there is a natural temporal metric: breathing. The ongoing process of breathing couples stimulus delivery to inhalation, and one can ask if the timing of neuronal activity with respect to this cycle is informative. Studies using various approaches from several labs indicate that the answer is positive. That is, consideration of neuronal activity with respect to the breathing cycle provides more information than is available by simply counting all spikes during an entire breathing cycle. In the VNS, there is no obvious ongoing cycle, but this does not preclude the relevance of temporal coding. If one considers the vomeronasal analogue of the sniff to be stimulus uptake, then we can ask whether neuronal response dynamics relative to the initiation of uptake by the VNO is informative.
To address this question, we have recently examined whether consideration of “fine” temporal dynamics provides more information than does consideration of the number of spikes generated within a broad window (e.g. firing rate over a long time window). In the figure below, we show the success of various temporal scenarios (which differ in the duration and the number of temporal windows) in decoding stimulus information. Each of the decoding schemes has its advantage and its costs. Perhaps the simplest scheme is to count the number of spikes emitted since stimulus uptake. Using this approach (cumulative single window, green line in panel B), for this stimulus set, stimulus identity can be detected in ~70% of the cases after about 5 seconds. To achieve the highest performance (~83%) using this scheme, it is required to sample for about 20 seconds. Slightly better performance can be obtained by considering a smaller window that begins after stimulus onset (red lines in panel C).
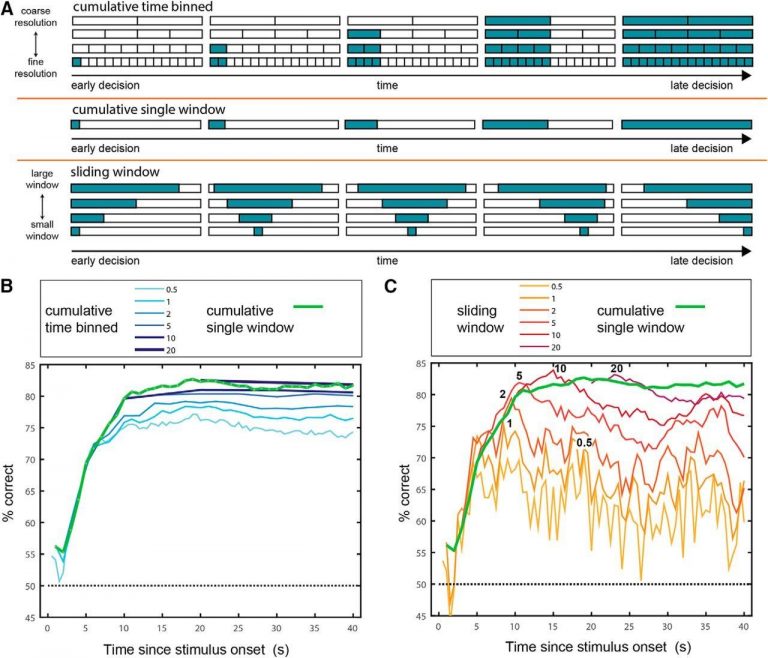
Performance of various decoding schemes using AOB mitral tufted cell responses.
All the temporal windows described above require knowledge of the time of stimulus onset. While it is very feasible that this information is available to neuronal decoders of AOB activity, due to the nature of VNO stimulus uptake, sampling is highly temporal variable. We thus asked if AOB responses can be decoded without knowing the time of stimulus onset. We found that this scheme, which we called time invariant decoding, can provide above-chance performance, for windows as small as 0.5 s. We also found that multiple neurons are required for this decoding scheme. In other words: multiple neurons are required not only to overcome variability in the properties of the stimulus, but also in the temporal domain of the responses (Panel A below). Panel B below illustrates the tradeoff between integration window duration, number of neurons in the population, and the decoding performance. Expectedly, more neurons and longer integration windows result in better decoding.
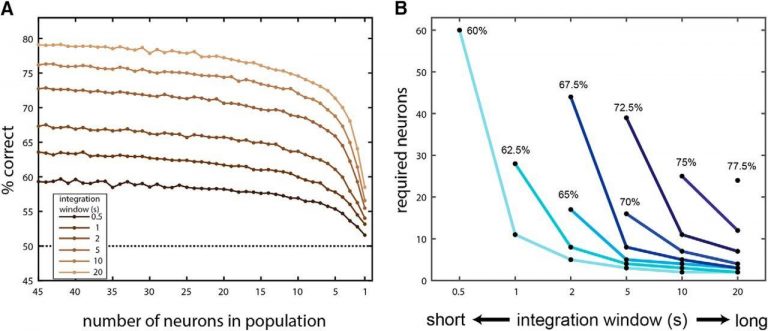
The time invariant decoding scheme.
Putting the details aside, these analyses suggest that fine temporal dynamics is not a key feature of AOB activity. This makes sense (to us), because VNS stimulus uptake is inherently a slow process. Why is uptake slow? Ultimately, the requirement to sample quickly or slowly depends on the dynamics of the stimuli. Unlike say the pressure waves that fluctuate at frequencies of ~10K Hz to create sound, the composition of molecules in urine or other bodily secretions does not change rapidly, and there is no benefit in very rapid sampling (which in this case is also constrained by the nature of the stimulus). If stimuli change slowly, then slow firing rates suffice to convey the information (fast rates are required for transmission of quickly changing stimuli), and if slow firing rates can convey the relevant information, there is no benefit to employ costlier firing rates. Indeed, AOB neurons do not exhibit very high firing rates, and given the nature of the stimulus and its uptake, this probably does not impose a bottleneck in the transmission of chemosensory information to deeper brain regions. We are now extending this work, and using optogenetics, to study which temporal aspects of AOB neuronal activity are available to make discriminations among stimuli.
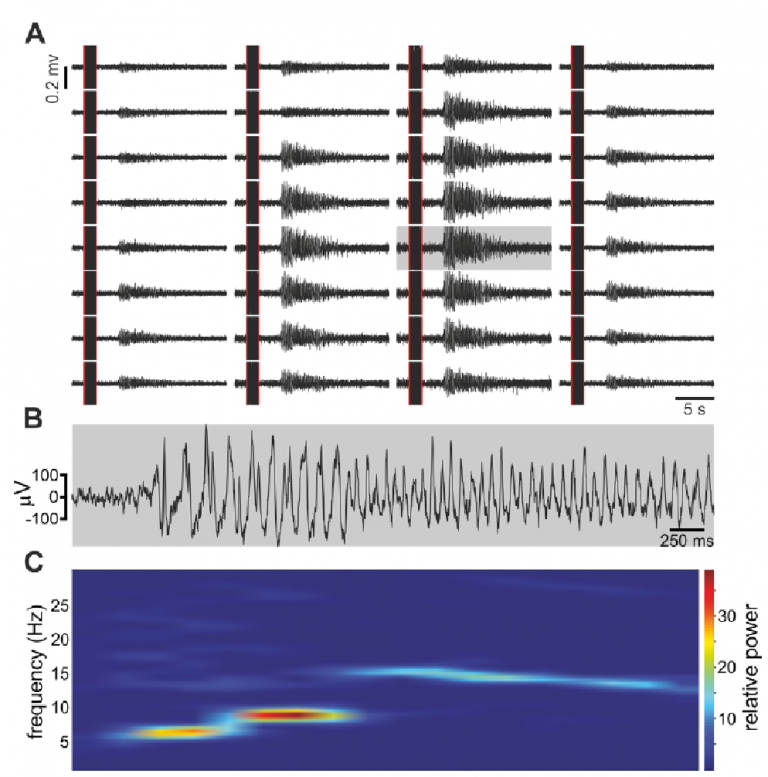
Another line of study in the lab, also related to the temporal dimension involves neuronal oscillations in the AOB:
Oscillations in the OB
As mentioned above, our analyses of VNS temporal dynamics were inspired by studies on the main olfactory system. A related set of studies in the main olfactory system examines the roles of oscillations, and particularly, population oscillations as expressed by the local field potential (LFP) signal. There are various patterns of main olfactory system LFP oscillations, each likely involving a distinct set (or sets) of neurons. This implies that the different patterns vary in their waveform characteristics, spatial loci, and their correlation with particular functional aspects. While it is still not clear what function, (if any…), these oscillations serve, they can probably tell us something about the processes that underlie chemosensory information processing. Several studies looked at LFP oscillations in the AOB, but none were clearly related to stimulus delivery. Recently, we have found such oscillations in our own recordings in anesthetized mice, and these are very similar to oscillations described by other groups. Currently, we are working to better characterize these oscillations and their relationship to stimulus delivery. In parallel, with our colleagues from the Spehr group, we are trying to understand the mechanisms underlying these oscillations.
Funding sources for these projects include the European Union (Marie Curie), Israeli science foundation, Israeli American Binational Science Foundation (BSF), German Israeli Science foundation (GIF) and DFG (deutsche forschungsgemeinschaft).
Ben-Shaul, Y., Katz, L. C., Mooney, R., & Dulac, C. (2010). In vivo vomeronasal stimulation reveals sensory encoding of conspecific and allospecific cues by the mouse accessory olfactory bulb. Proceedings of the National Academy of Sciences, 107(11), 5172-5177.
Our work is described in rough chronological order, starting with my postdoctoral work. This paper, describing my work with the late Larry Katz, Richard Mooney, and Catherine Dulac, describes our first in vivo recordings from the mouse accessory olfactory bulb (AOB). The methodology developed in this work is still practiced in our lab, and many of the themes of our present research are already present in this work.
Bergan, J. F., Ben-Shaul, Y., & Dulac, C. (2014). Sex-specific processing of social cues in the medial amygdala. Elife, 3, e02743.
In this work, part of my postdoc with Catherine Dulac, Joe Bergan and I extended my earlier postdoctoral work and recorded responses in the medial amygdala. We have shown a higher level of selectivity as compared to the AOB, and more strikingly, very clear evidence for sexual dimorphism.
Ben-Shaul, Y. (2015). Extracting social information from chemosensory cues: consideration of several scenarios and their functional implications. Frontiers in neuroscience, 9, 439.
The first publication from my own lab is a theoretical discussion of the conceptual difficulties of deriving meaningful and invariant information from chemical signals. These thoughts are very much related and inspired by the data described in the following paper by Anat Kahan.
Kahan, A., & Ben-Shaul, Y. (2016). Extracting behaviorally relevant traits from natural stimuli: benefits of combinatorial representations at the accessory olfactory bulb. PLoS computational biology, 12(3), e1004798.
This is the first experimental work from the lab. Work of Anat Kahan in which we explored how information about a female’s reproductive state and strain are encoded by activity of AOB neurons. In agreement with the Frontiers paper above, we show that extraction of information requires multiple neurons.
Yoles-Frenkel, M., Cohen, O., Bansal, R., Horesh, N., & Ben-Shaul, Y. (2017). In vivo stimulus presentation to the mouse vomeronasal system: surgery, experiment, setup, and software. Journal of neuroscience methods, 285, 19-32.
This is a methodology paper, in which we describe, in detail, the in vivo recording approach that we use in the lab.
Yoles-Frenkel, M., Kahan, A., & Ben-Shaul, Y. (2018). Temporal response properties of accessory olfactory bulb neurons: limitations and opportunities for decoding. Journal of Neuroscience, 38(21), 4957-4976.
In this paper, we examined the benefits of using fine temporal metrics for decoding stimulus features from activity of neurons in the accessory olfactory bulb (AOB). We have shown that in line with the very slow time course of vomeronasal system activity, activity of AOB is temporally imprecise, and does not support readout code with high temporal resolution. This is in contrast with the main olfactory system.
Ben-Shaul, Y. (2017). OptiMouse: a comprehensive open source program for reliable detection and analysis of mouse body and nose positions. BMC biology, 15(1), 1-22.
This is another Methodological paper, describing software that we developed to analyze mouse positions in an arena. Although these days the field is dominated by powerful code that uses deep neural networks (i.e. deeplabcut), we still believe that for some tasks and for some setups, OptiMouse remains a good solution.
Marom, K., Horesh, N., Abu-Snieneh, A., Dafni, A., Paul, R., Fleck, D., … & Ben-Shaul, Y. (2019). The vomeronasal system can learn novel stimulus response pairings. Cell Reports, 27(3), 676-684.
In this paper we challenged one of the main (implicit) assumptions about vomeronasal system function, which is that it is a rigid system that cannot be readily used to form new stimulus-response associations. Using optogenetics and behavioral analysis, we show that this assumption is false.
Bansal, R., Nagel, M., Stopkova, R., Sofer, Y., Kimchi, T., Stopka, P., … & Ben-Shaul, Y. (2021). Do all mice smell the same? Chemosensory cues from inbred and wild mouse strains elicit stereotypic sensory representations in the accessory olfactory bulb. BMC biology, 19(1), 1-19.
The meaning of the title of this paper is intentionally ambiguous (is it smell as in the action of smelling, or to smell as in to have a certain odor). Following the first interpretation, we compared neuronal representations in the accessory olfactory bulbs of two different strains of mice. We showed that they are very similar, despite variations associated with their distinct genetic backgrounds, experience, and mouse to mouse variability. Following the second interpretation, we compared responses to urine stimuli from inbred and wild mice. Perhaps surprisingly, almost all physiological data of responses to chemosensory stimuli is based on exposure to stimuli from inbred mice. Here, we showed that in many aspects inbred and wild derived mouse stimuli elicit similar responses. There is however, an interesting difference between secretions of wild derived mice (wild mice bred several generations in the laboratory) and wild mice
Yoles-Frenkel, M., Shea SD., Davison IG., Ben-Shaul, Y. (2022). The Bruce effect: Representational stability and memory formation in the accessory olfactory bulb of the female mouse. Cell Reports 40(8).
This paper marks the end of a long journey that began during my postdoc. In fact, studying the neuronal correlates of the Bruce effect (BE) was my original postdoc project. It turned out that addressing this question is more complex than I initially realized, and it was only about 10 years after starting our lab, that Michal Yoles-Frenkel, a PhD student, was able to address this question. In the BE, a mated female mouse becomes resistant to the pregnancy-blocking effect of the stud. Various lines of evidence suggested that this form of behavioral imprinting results from reduced sensitivity of the female’s accessory olfactory bulb (AOB) to the stud’s chemosignals. However, the AOB’s combinatorial code implies that modifying responses to one individual will distort representations of other stimuli, and this would clearly pose a problem. To test the nature of representations following mating, Michal recorded extracellular activity of AOB neurons in mated and unmated female mice while presenting urine stimuli from the stud male and from other sources. To make a long story short, several types of analyses revealed that sensory responses in the AOB, both to the stud male and to other stimuli, remain stable. While we were close to concluding that we find no evidence for plasticity in the AOB following mating, we decided to conduct one more set of experiments, inspired by findings from our colleagues (Gao et al. eLife 2017). In these experiments, Michal applied a prolonged stimulation protocol, mimicking the extended time course required for eliciting the pregnancy block in behaving mice. These experiments supported the idea that mating leads to a selective reduction under a prolonged presentation of mating male cues. Michal’s findings, and specifically, the temporal disassociation between slow and fast responses could allow attenuation of slow-acting endocrine processes in a stimulus-specific manner without compromising ongoing representations that are needed to guide behavior.
Oksana Cohen, Anat Kahan, Idan Steinberg, Sebastian Malinowski, Dan Rokni, Marc Spehr and Yoram Ben-Shaul (2023). Stimulus-induced theta band LFP oscillations format neuronal representations of social chemosignals in the mouse accessory olfactory bulb. Journal of Neuroscience
The AOB is the first central stage of the vomeronasal system, a chemosensory system dedicated to processing cues from other organisms. Information from the AOB is conveyed to other brain regions via activity of its principal neurons, mitral cells (AMCs). Here, we show that socially relevant sensory stimulation of the mouse vomeronasal system leads not only to changes in AMC activity, but also to distinct theta band (∼5 Hz) oscillatory episodes in the local field potential. Notably AMCs favor the negative phase of these oscillatory events. Our findings suggest a novel mechanism for the temporal coordination of distributed patterns of neuronal activity, which can serve to efficiently activate downstream processing stages.
Yisraeli, E., Elizera, Y., & Ben-Shaul, Y. (2025). Regularity, variability, and individuality: urine marking patterns of male mice towards stimuli representing varying degrees of kinship. Chemical Senses, bjaf028.
Successful social interactions require the identification of conspecifics and their traits. Often, individuals do not directly interact with conspecifics, but rather with their secretions. Urine provides a wealth of social information, and accordingly, several species, including mice, use it to advertise and mark territories. Here, we asked if kinship relations are reflected by the subject’s marking patterns. Specifically, we studied counter-marking patterns of outbred ICR male mice following presentation of urinary cues from conspecifics with varying degrees of kinship. Examination of more than 1000 individual marking patterns from 10 mice reveals a high degree of variability. Variability is apparent across different mice and across single marking bouts of any given individual. Yet, we identify consistent effects of stimulus kinship, and, somewhat unexpectedly, even more robust differences among individuals. Individual-specific marking patterns are also evident in an empty arena, prior to the introduction of an external stimulus. Stimulus presentation gives rise to further changes in marking patterns, reflecting the relationship between the subject and donor mice. Notably, while stimuli representing highly distinct kinship relations induce robust differences at the population level, finer distinctions, including discrimination of same-strain conspecifics and self-urine, are only displayed by a subset of mice. Thus, while counter marking patterns are determined by a variety of factors, some of which cannot be easily controlled or measured, they ultimately reflect the identity of the marker and the kinship relation with the stimulus donor.
Cohen, O., & Ben-Shaul, Y. (2025). Representation of male features in the female mouse Accessory Olfactory Bulb, and their stability during the estrus cycle eLife.
This work substantially advances our understanding of how accessory olfactory bulb neurons respond to social odor cues across the estrous cycle, showing that responses vary with the strain and sex of the odor source but display no consistent differences between estrous and non-estrous states. It employs a unique electrophysiology preparation that activates the vomeronasal organ pump via electric stimulation, enabling precise recordings of accessory olfactory bulb cell responses to different chemosignals in anesthetized mice. Overall, the study presents convincing findings on the stability and variability of accessory olfactory bulb response patterns, indicating that while accessory olfactory bulb detects social signals, it does not appear to interpret them based on reproductive state. This work will be of interest to those studying olfaction, social behavior, reproductive cycles, and systems neuroscience more broadly.
Cichy, A., Ackels, T., Tsitoura, C., Kahan, A., Gronloh, N., Söchtig, M., … & Spehr, M. (2015). Extracellular pH regulates excitability of vomeronasal sensory neurons. Journal of Neuroscience, 35(9), 4025-4039.
A collaboration with the Spehr lab who led this study, showing and analyzing the mechanistic basis of pH sensitivity of vomeronasal system sensory neurons. Our contribution was to provide data showing that AOB neuronal responses can also be pH dependent.
Zylbertal, A., Kahan, A., Ben-Shaul, Y., Yarom, Y., & Wagner, S. (2015). Prolonged intracellular Na+ dynamics govern electrical activity in accessory olfactory bulb mitral cells. PLoS biology, 13(12), e1002319.
A collaboration with the group of Shlomo Wagner from Haifa University who led this study, and with Yossi Yaron at HUJI, analyzing the underlying mechanisms of prolonged responses in mitral tufted cells of the mouse AOB
Gorin, M., Tsitoura, C., Kahan, A., Watznauer, K., Drose, D. R., Arts, M., … & Spehr, M. (2016). Interdependent conductances drive infraslow intrinsic rhythmogenesis in a subset of accessory olfactory bulb projection neurons. Journal of Neuroscience, 36(11), 3127-3144.
Another collaboration with the Spehr lab, providing the first report of the rhythmic patterns of responses of AOB neurons. Following the observations made in vitro by Monika Gorin and Chryssanthi Tsitoura in the Spehr lab, we have analyzed data from Anat Kahan, and found similar patterns of slow oscillations in vivo as well.
Rokni, D., & Ben-Shaul, Y. (2024). Object-oriented olfaction: challenges for chemosensation and for chemosensory research. Trends in Neurosciences.
Many animal species use olfaction to extract information about objects in their environment. Yet, the specific molecular signature that any given object emits varies due to various factors. Here, we detail why such variability makes chemosensory-mediated object recognition such a hard problem, and we propose that a major function of the elaborate chemosensory network is to overcome it. We describe previous work addressing different elements of the problem and outline future research directions that we consider essential for a full understanding of object-oriented olfaction. In particular, we call for extensive representation of olfactory object variability in chemical, behavioral, and electrophysiological analyses. While written with an emphasis on macrosmatic mammalian species, our arguments apply to all organisms that employ chemosensation to navigate complex environments.

State-dependent processing in the vomeronasal system
Cognitive principles of navigation in the social world

PhD student
I study the relationship between innate and learned behaviour, in the context

M.Sc student
researching how mice identify individuals in their social circle

M.Sc student
The effect of odor on urinating patterns in mice

Postdoc
Structure function analysis of accessory olfactory bulb neurons
Current location
China

postdoc
In the Ben-Shaul lab, I looked at temporal and combinatorial aspects of the accessory olfactory bulb in sex and strain discriminations.
Current location
California Institute of Technology

Lab Manager
MSc student for Neurobiology in Biomedical Sciences
Current location
Lichtstein lab – Hebrew university faculty of medicine

ELSC graduate student
Temporal coding in the vomeronasal system, Imprinting in the context of the Bruce Effect
We are recruiting students and postdocs who share our research interests. The key requirements are enthusiasm for the work we are doing and a background in neuroscience.
Our main goals are to understand how chemosensory cues are processed by the neuronal circuits of the vomeronasal system, how this processing leads to particular behavioral/physiological outcomes, and how it is modified by current state and experience. Our primary research approach is electrophysiological recordings (mostly extracellular, but also intracellular) from vomeronasal system structures upon controlled stimulus delivery in anesthetized mice. This approach allows us to quantify neuronal responses in various brain regions under various states.
In addition, we use behavioral analyses (primarily to assess preference and associative learning), as well as optogenetic and molecular approaches to manipulate particular neuronal populations.
A crucial aspect of our work is data analyses. Freedom to ask questions requires proficiency in programming, and we routinely use MATLAB in the lab to process and analyze our data.
Though clearly helpful, previous experience in in vivo electrophysiology, mouse behavior, and programming are not prerequisites, but ability and interest to study these approaches is.
I majored in Biology at the Hebrew University, and then continued to a PhD in computational neuroscience at the ICNC (interdisciplinary center for neuronal computational, now ELSC) working on encoding of complex movements in primates at the lab of Moshe Abeles. I then did a brief postdoc with Hagai Bergman and Hermona Soreq where I transitioned to mouse work, and learned and applied molecular and bioinformatic approaches to a mouse model of Parkinson’s disease. I then continued for a postdoc with Larry Katz at Duke and Catherine Dulac at Harvard. It was during this postdoc I started working on the vomeronasal system which remains the subject of our research. In our lab, we aim to combine computational and experimental approaches to better understand information processing in the context of social chemo-signaling.